Category Archives: News
Best known for their versatile role in a vast array of chemical applications, organic solvents play a crucial role in numerous sectors, including the medical industry.
To gain a better understanding of how these solvents can contribute to pharmaceutical practices and manufacturing techniques for drug delivery, review our complete guide for more information.
An Overview of Organic Solvents
As industry experts are aware, organic solvents are solvents that contain carbon atoms capable of either dispersing or dissolving one or more substances. Due to their chemical makeup, these solvents can be utilized in a variety of industries, such as textiles, cleaning, agriculture, and pharmaceuticals.
Additionally, common characteristics and properties of suitable organic solvents include the following:
- High volatility
- Acid-based properties
- Low boiling point
- Suitable density
As opposed to some inorganic solvents, organic solvents can also be described as combustible and extremely flammable. That’s why we advise that those looking to work with organic solvents in long-acting injectables, or processes such as spinning and dyeing textiles, ensure that they are handling these carbon-based solvents with the utmost care.
The Role of Organic Solvents in Long-Acting Injectables
Organic solvents in pharmaceuticals, such as long-lasting injectables, can contribute to an expansive array of roles throughout the drug delivery process, such as the following:
- Dissolving drugs at high concentrations – Organic solvents can be utilized to dissolve drugs, such as biologics, that otherwise have a higher concentration and a lower aqueous solubility. That way, long-acting injectables can be administered at the appropriate viscosity and form drug depots under the skin’s surface.
- Forming drug depots – Drug depots, or depot injections, are longer-duration drugs that result in a slow absorption into the bloodstream. Though organic solvents can contribute to the formation of these drug depots, the administration of the drug into your muscle is best when using a depot.
- Determining the rate of release – Once the drug depot has formed, organic solvents decelerate the rate of their delivery, resulting in a more gradual, sustainable release and absorption of the medication over time.
A Look at Organic Solvents in Pharmaceuticals: Their Industry Uses and Beyond
Along with their inherent contribution to the production of long-lasting injectables, organic solvents in pharmaceuticals play many key roles in the total industry. Beyond mainly being utilized as reaction media, organic solvents are used in the following processes:
- The purification of synthesis products
- Excipients, such as coloring agents, preservatives, and fillers
- The bioavailability of orally administered protein-active sites
- Semiempirical models of meditated solubility
- Antisolvent crystallization techniques in pharmaceutics
Additionally, organic solvents can even be used in water-based cleaning solutions. This is because they are capable of penetrating oily soil deposits while also dissolving in water.
FAQs About Organic Solvents, Their Capabilities, and More
What are the most common organic solvents used in pharmaceutical manufacturing?
The most common applications when working with organic solvents in pharmaceuticals include the following:
- Acetone
- Dimethylformamide
- Diethyl ether
- Benzene
- Isopropanol
- Ethanol
What safety precautions should be taken when working with organic solvents in pharmaceuticals?
As stated previously, organic solvents can be extremely flammable and volatile, so handling them with care is essential. With that in mind, here are some stringent safety regulations you should abide by during the manufacturing process:
- Gloves and safety glasses should be worn when handling the materials
- A fume-hood is required when pouring out organic solvents
- Spill kits and eyewash stations should be readily available in the workspace
- Flammable liquids, such as bleach, peroxides, and acids, should be stored away from organic solvents
Can organic solvents be reused or recycled?
Yes, organic solvents can be recycled easily and reused in syntheses. This includes collected solvents that are separated by distillation and have different boiling points.
Contact Oakwood Labs for More Information
As your trusted resource for sustained-release injectables and other services, Oakwood Labs is well-equipped with the necessary tools to satisfy your application needs.
For more information about organic solvents in pharmaceuticals, long-acting injectables, and more, please reach out to our team today. We look forward to assisting you.
Long-acting injectables are favored in the medical field due to their effectiveness and convenience, as their injection methodology allows for the gradual release of medication into the bloodstream. In post-operative care, the alleviation of pain is vital not only for recovery, but for the comfort of the patient. With the help of long-acting injectable drugs for post-surgical pain management, patients can experience a more effective recovery.
The History of Long-Acting Injectables
Long-acting injectable drugs were first introduced as a treatment for people with chronic schizophrenia. These long-acting injectable antipsychotic drugs were created as an effort to solve the issue of poor adherence to oral medications. Some notable points in the history of long-acting injectables include the following:
- 1950s – 1970s – Long-acting injectables were created to help manage conditions such as schizophrenia. In 1952, chlorpromazine (CPZ) was developed in Paris, offering an effectiveness greater than that of older drugs on the market. Then in 1966, thanks to G.R. Daniels, a medical director at the time, the first long-acting injectable was created. This first injectable was AP fluphenazine enanthate, with fluphenazine decanoate being created just eighteen months later.
- 1980s – 1990s – During this time, long-acting injectables for second-generation antipsychotics were introduced. Between 1988 and 1992, Risperidone was first developed by Janssen-Cilag. Its purpose was to treat behavior issues in children and adolescents.
- 2000s – present – Long-acting injectables have come a long way since their creation. With their medication methodology, they can provide better adherence to timelines, facilitate proper dosing, and free patients from having to take oral medications every day. With continuing research on long-acting injectables, we are seeing uses in areas such as contraception, chronic and acute pain, antiretroviral therapy, and more.
The Role of Long-Acting Injectables for Post-Surgical Pain Management
A key factor of post-surgical work is minimizing patient pain, giving them the time they need to heal. With the use of extended-release drugs for post-surgical pain management, doctors are better able to effectively manage pain for their patients and promote an expedited recovery process.
Types of Injectables Used for Post-Surgery
NSAIDs, or nonsteroidal anti-inflammatory drugs, are medications that reduce fever, pain, and inflammation. Looking at postoperative care, NSAIDs help in mitigating pain, minimizing the reliance on opioids, and fostering a healing environment. You may be familiar with some over-the-counter NSAIDs such as ibuprofen or aspirin.
NSAIDs are known to prevent blood from clotting, which can definitely be beneficial in post-surgery situations. In cases such as orthopedic procedures that involve prolonged immobility, there is an increased risk of blood clot formation. NSAIDs help prevent that blood clotting, specifically in the veins.
Some common NSAIDs that have been used as long-acting injectables for post-surgery pain management include:
- Toradol (Ketorolac) – This NSAID is used to relive pain and is commonly available in injection or tablet form. The injections work about thirty minutes after administration and the effects can last up to six hours. Often prescribed for post-surgery pain, it typically is distributed as a one-time injection. However, some people may receive a shot every six hours. This will depend on factors such as body weight, age, drug interactions, and others.
- Diclofenac sodium – This medication is another NSAID used to treat adults with mild to moderate pain. It can be used for osteoarthritis, low back pain and for post-operative patients. Similar to Toradol, the effects may start to work immediately after the injection.
- Caldor – This ibuprofen injection works by reducing fever and treating pain in the body. It helps manage pain as an adjunct to opioid analgesics.
- Ketoprofen – Ketoprofen can help with acute and chronic pain, both for the traumatic and rheumatic origin. Additionally, it can help alleviate postoperative pain, specifically in the orthopedic field. While ketoprofen is offered as an injectable, that is not the intended use for humans. There is an oral tablet for humans, only available with a doctor’s prescription. The injectable form is actually intended for animals, such as horses. In horses, it’s used for musculoskeletal pain or inflammation, abdominal pain, and other inflammatory conditions. It can also be used effectively for cattle, goats, sheep, and pigs.
The Capabilities of Oakwood Labs
At Oakwood Labs, we are a leading developer of sustained-release pharmaceuticals and have over 20 years of experience in drug encapsulation. We are here to offer support throughout the development of different types of long-acting injectables.
Our facilities can provide the following to help you achieve your project goals:
- GMP manufacturing (commercial and aseptic)
- Pre-formulation development
- Phase I, II, and III clinical trial manufacturing
- Scaling formulation
- ICH compliant stability tests
- Feasibility studies
- Toxicology batch manufacturing
Contact Us to Learn More
With our history of compliance and commitment to quality, we continue to be a leader in injectable pharmaceutical development. If you’re wanting to learn more about injectable development or our other capabilities, contact us today.
Knee pain is a common issue for adults because it is caused by general stress from normal daily activities. In fact, around 25% of U.S. adults experience knee pain, and many of them require treatment to make day-to-day activities easier.
Long-acting injectables are able to target specific anatomical areas like eyes, ears, knees, and other spots to relieve pain and reduce systemic exposure. This means they are capable of providing a means of comfort for patients experiencing problems like osteoarthritis, rheumatoid arthritis, bursitis, or tendonitis. Learn more about the benefits of extended-release drugs for knee pain and how you can help patients by manufacturing them.
Benefits of Using Extended-Release Drugs for Knee Pain
Extended-release drugs and long-acting injectables for knee pain are designed to release the active ingredient over time. These medications encourage the maintenance of the therapeutic levels in the body for an extended time period and provide prolonged pain relief. The outcome of prolonged pain relief is less frequent dosages and happier patients.
Other benefits of using these types of medication include:
- Reduced side effects – Extended-release drugs encourage lower overall doses when compared to the immediate-release competition. This can lead to fewer side effects, as the patient will be exposed to a lower peak concentration of the drug.
- Improved patient adherence – Since these types of drugs require less frequent doses, patients are more likely to adhere to their prescribed regimen. This can lead to better pain management outcomes, making them the go-to choice for more patients.
- Minimized fluctuations in pain levels – When patients are given immediate-release formulations they may experience highs and lows from the drug concentration that leave them with fluctuations in the extremity of their knee pain. Using extended-release medications for knee pain limits this issue by providing a consistent and controlled release.
Types of Extended-Release Drugs for Knee Pain
There are currently two main types of extended-release injectable drugs for knee pain. The type that is administered will depend on the patient’s problem, pre-existing health conditions, and more.
Corticosteroid Injections
Corticosteroid injections reduce pain and inflammation in the knee joint, thanks to their powerful anti-inflammatory agents. These synthetic drugs mimic the effects of natural hormones produced by adrenal glands. The relief is often found quickly, with many patients experiencing reduced pain in just a few days. The relief should last around three to four months, depending on the patient and the severity of their condition.
A prominent example of a corticosteroid long-acting injectable for knee pain is inserting Triamcinolone LAI in the knee. Another common type is ZILRETTA®.
Hyaluronic Acid Injections
Another type of long-acting injectable drug for knee pain is hyaluronic acid injections. Known for their ability to replenish knee lining and reduce friction on the joints, hyaluronic acid injections are an extremely effective treatment for those fighting osteoarthritis. These injections also protect the cartilage and bone from further damage, relieving pain effectively. While they may not have the longevity of a typical long-acting injectable drug for knee pain, they will provide patient relief for between two and six months.
Hyaluronic knee injections currently on the market include Durolane, Euflexxa, Hyalgan, Monovisc, and more.
Using Best Practices When Manufacturing Long-Acting Injectables for Knee Pain
Adhering to best practices when producing long-acting injectable drugs for knee pain is essential, and it’s something the team at Oakwood Labs is committed to. Doing so assures your patients that the solution was created using rigorous processes and that the medication is safe.
Let’s discuss some of the best practices for creating extended-release drugs for knee pain and how Oakwood Labs can provide.
- Conduct clinical research and development – The process starts with conducting pre-clinical studies to assess feasibility, safety, and efficacy. Then, it is time to study the population to create the intended patient demographic, as well as execute clinical trials to ensure that the solution is relevant to the problem and population.
- Create optimal formula – The next step is to research and develop a formulation that will provide sustained and effective pain relief without complications or introducing new side effects. Be sure to check this over time and ensure that the extended-release drug for knee pain will continue to provide the most benefits to the patient.
- Determine dosing and administration – Based on clinical research results, you will want to determine an appropriate dosage and administration schedule. Then, create clear and concise instructions for proper administration for patient safety and reduced risk.
- Assess safety – Once you develop the proper instructions for administering the long-acting injectable for knee pain, it is important to conduct comprehensive safety assessments that define adverse effects, drug interactions, and any risks. Then, be sure to monitor unexpected side effects during post-marketing surveillance.
- Provide education and training – Collect comprehensive educational materials that healthcare professionals and patients can look at to ensure the best understanding of the long-acting injectable for knee pain as well as any side effects.
- Adhere to ethics – Ethics are a priority in pharmaceutical manufacturing, covering everything from reporting results to ensuring equal access. Be sure to adhere to ethical standards in all aspects of development.
- Choose a GMP manufacturer – Oakwood Labs operates in an FDA-approved cGMP manufacturing facility that is capable of conducting Phase I, II, and III clinical materials, delivering commercial products, and more. We also have decades of industry experience, making us the ideal destination for manufacturing long-acting injectables for knee pain. As your partner, we can offer short lead times, robust systems, CMC development capabilities, and so much more.
Contact Us to Learn More
If you are interested in manufacturing extended-release drugs for knee pain, Oakwood Labs would be happy to partner with you.
When it comes to drug creation and formulation, ensuring that both the creator and the property are protected is key. That is why it is important to understand what your intellectual property rights are, and how to defend them even after FDA review and distribution.
With that in mind, check out our informational guide on intellectual property protection, and how it applies to the pharmaceutical industry.
An Overview of Intellectual Property Protection
Intellectual property protection was established to legally safeguard ideas and inventions of the mind. This includes original pieces of work, such as artistic creations, symbols, names, manufacturing processes, pharmaceutical formulas, and beyond.
Intellectual property also comes in various forms depending on the medium of the work. The main types that you should be familiar with relate to the following:
- Patents
- Data exclusivity
- Trade secrets
- Copyright
- Trademarks
- Regulatory exclusivity
Why Is Intellectual Property Protection Vital in the Pharmaceutical Industry?
The protection of intellectual property in the pharmaceutical industry allows pharmaceutical creators to benefit fiscally from both idea fabrication and product manufacturing. Additionally, some of the reasons that intellectual property is legally protected and closely monitored include the following:
- Promotes continuous innovation – Since patents and other intellectual property types provide outside investors with the motive to put money into the research phase of drug creation, pharmaceutical scientists can continually work on evolving existing developments. Along with manufacturing and distributing current drugs, scientists can also create new drugs and start desired projects while knowing that their progression is being fully supported financially.
- Increases economic growth – Due to the ongoing process and competition that comes with bringing new drugs to the market, the creation of pharmaceuticals can produce further economic opportunities, such as generating new jobs and boosting overall exports on a global scale.
- Prevents misuse and confusion – With intellectual property protection, the rights to the formula and branding of a product belong solely to the company or individual who created it. That means consumers will know exactly who and where the product has come from, as well as what the intention of the specific product is. This will avoid misuse of the product itself, as well as confusion about the specifications of the product.
Overall, the protection of intellectual property increases innovative initiatives for companies looking to cultivate and expand upon drug development ideas and procedures. Alongside the developmental period for pharmaceuticals, intellectual property measures also legally protect creators from property infringement by competitors, ultimately boosting the production, distribution, and consumption of necessary medications.
A Brief History of Pharmaceutical Intellectual Property Protection
When it comes to protecting the creation of new drugs, applications, and research, intellectual property protection is crucial. The origins of intellectual property are not new ideas either, as the first instances of intellectual property disputes and one-year patent statements date back as far as 500 B.C.E.
In the modern era, there have been numerous cases that have shaped how we navigate property ownership and distribution. Alongside those cases, here are just some of the notable historical events that have progressed intellectual property rights:
- 1928 – Alexander Fleming discovered penicillin at St. Mary’s Hospital in London. This discovery marked the beginning of the antibiotic age, which lasted until around 1970, with 1940 to the early 1960s being considered the true golden age of the antibiotic era. During this time as well, product patents for drugs became more prevalent as growth and expansion prospered, though global drug patents were first introduced for the pharmaceutical industry in the late 17th century.
- 1984 – The Hatch-Waxman Act was established in the United States, laying the foundation both legally and economically for the U.S.’s generic pharmaceutical industry. And as more countries, such as Canada, began to recognize the importance of drug patents as well, multinational corporations (MNCs) within the pharmaceutical industry started to advocate on a global level for stronger intellectual property protection and patent laws for their products.
- 1994 – The TRIPS Agreement was established in this year and set minimum enforceable standards for various forms of intellectual property across numerous industries. For the pharmaceutical industry, it required countries part of the World Trade Organization (WTO) to make patents available for any products or processes without discrimination, completely replacing the “process patent” system that was in place at the time.
- 2000 onward – Patent thickets, evergreening, and consolidation practices become highly common in the pharmaceutical industry. Also during this time, we saw large lobbying efforts in the United States to try to prevent price controls from stopping skyrocketing prices on necessary drugs. This includes key bills, such as The Inflation Reduction Act of 2022.
FAQs – Additional Aspects to Consider
For more of an in-depth look at intellectual property protection and how to navigate the main types, check out the answers to some of our most commonly asked questions.
How long do patents last on new drugs?
As of this writing, patents last 20 years from the filing date listed on the patent application. Once a drug patent expires, manufacturers other than the original developer have the freedom to produce generic versions of the product. These drugs are usually clinically equivalent to the original.
Can generic drugs have the same trade name as the original drug?
No. Though the chemical composition may be almost exactly alike, trademarks protect the original brand name, and generic brands must accommodate these regulations to avoid consumer confusion.
Does intellectual property protection increase drug prices?
Yes, it can potentially lead to an increase in drug prices. This is because patent exclusivity both delays cheaper versions of a drug from being made, as well as allows drug companies to have complete control over the pricing of their products.
How are manufacturing processes and drug formulas protected today?
One of the key ways drug formulas and their processes are protected is via trade secrets, which provide companies with a competitive advantage. These secrets do not need to be registered and can be protected for an unlimited amount of time, though there are some conditions for protection, including the following:
- The information must not be known to the general public
- The formula or process must have commercial value to be secret
- Reasonable steps must be taken by the information holder to keep it secret
Contact Oakwood Labs Today to Learn More
Have any additional questions? If so, reach out to our team at Oakwood Labs today. We look forward to answering your questions or discussing our services with you.
Oakwood Labs is proud to announce that we will be at the 2023 Controlled Release Society 2023 annual meeting and expo, July 24-28, 2023. The theme this year is “The Future of Delivery Science,” which focuses on the next generation of scientists and progressive research that is being conducted around the world.
Find Us at Booth 304
As a global leader in sustained-release drug delivery, Oakwood Labs is able to solve changes related to developing and manufacturing pharmaceutical injectables. We pride ourselves on being transparent with our clients throughout the whole process and offering our expertise. If you’re looking for state-of-the-art technology, personnel, and equipment, you’ll want to check out Oakwood Labs at booth #304.
We can help you with the following:
- Clinical trial manufacturing (Phase I, II, III)
- Feasibility studies
- Pre-formulation development activities
- Commercial aseptic GMB manufacturing
- Scale-up of formulation
- Manufacturing toxicology batches
- ICH compliant stability testing
Contact Us for More Information
The CRS 2023 conference covers a wide range of topics, and we are happy to contribute to the event! If you aren’t able to attend, but still want to learn more about Oakwood Labs or what you missed at the conference, contact us today!
We look forward to discussing our technology and more.
Amino acids have proven their capabilities in the pharmaceutical industry in a variety of ways. They can be used alone as nutritional supplements, synthesized to help form other products, or even built into drug delivery systems.
Since Oakwood Labs focuses on drug formulation, we wanted to explain the role of amino acids in pharmaceuticals, specifically addressing how they impact sustained-release injectables and provide therapeutic benefits to patients.
How Amino Acids Benefit Your Pharmaceutical Projects
Amino acids are used in the development and function of sustained-release injectables and pharmaceuticals because they help with matrix formulation, suspension, drug diffusion, and biocompatibility.
Different amino acids and combinations can be deployed to achieve specific therapeutic goals. With this in mind, the amino acids used will depend on factors like the drug that is being delivered, the intended release kinetics, and the compatibility of the overall formation.
Using amino acids in pharmaceuticals like sustained-release injectables assist with the following:
- Matrix formulation – The use of amino acids in sustained-release injectables can help form a matrix that is modified to perform long-acting therapy for the patient. The matrix encapsulates the active drug and helps control the rate at which it is released, making it ideal for less frequent doses and better patient compliance.
- Solubility – Solubility is needed in drug formulations because it leads to effective absorption. By enhancing the solubility of certain drugs, amino acids better maintain the composition of the drug and promote absorption into the body.
- Stability – Along with solubility, amino acids also protect the drug from degradation throughout the process of storage and release. Stabilization additionally helps increase drug effectiveness, especially when dealing with sustained-release applications.
- Modulation – Using amino acids in a sustained-release application can adjust the overall drug output to meet the demands of the patient. This means amino acids are capable of altering the diffusion characteristics of the drug being used within the formulation to better meet a patient’s therapeutic objectives.
- Biocompatibility – Amino acids used in pharmaceutical applications like sustained-release injectables can promote biocompatibility for medical applications. Biocompatibility means that the injectable will not interfere with any of the other cellular functions or compromise the patient’s wellness, and it is important because it increases safety, long-term tolerability, and regulatory compliance.
Benefits of Using Sustained-Release Pharmaceuticals
Sustained-release injectables and pharmaceuticals have gained popularity in recent years due to their variety of benefits. Since the drug remains active in the bloodstream over a period of time, sustained-release dosages offer prolonged therapeutic effects. This helps improve patient compliance and reduce the frequency of dosing.
Other advantages of sustained-release dosages include:
- Prolonged action of the medicaments
- Control over drug therapy
- Ability to modify the extent and rate of drug absorption
- Delivering API directly to the desired location
- Decreasing total amount of a drug, reducing local side effects
- Ability to modify the extent and rate of drug absorption
Work with Oakwood Labs on Your Next Project
If you are looking to incorporate amino acids into your pharmaceuticals, be sure to request help from the team at Oakwood Labs. We are a leading manufacturer of sustained-release pharmaceuticals and will work with you throughout the creation process. This includes proof of concept, feasibility studies, clinical trials, and finally the production of an FDA-approved commercial supply.
Additionally, our team has over 20 years of experience, which makes us a trusted and effective partner for global pharmaceutical firms of all sizes. See how we can incorporate amino acids into your sustained-release pharmaceuticals and beyond today.
Learn More About Using Amino Acids in Pharmaceuticals
The team at Oakwood Labs is ready to help you achieve high-quality results in your next project. Our quality assurance and validation practices will deliver the best solutions, regardless of the formulation needed.
For more information on using amino acids in sustained-release pharmaceuticals, be sure to contact us today.
In the pharmaceutical field, molecules are often a major topic since they play a key part in research and development. Small and large molecules each have their place, but small molecules are particularly significant in drug development since their size allows for easy passage through the gastrointestinal tract.
Benefits of Small Molecule Injectables
Small molecule pharmaceuticals are low molecular weight compounds that can be administered in a variety of ways, including via intravenous (IV), intramuscular (IM), or subcutaneous (SC) injections. In addition to offering various ways to inject medicine, small molecule injectables also provide benefits such as:
- Rapid onset of action – Small molecules are able to be absorbed quickly into the bloodstream, allowing them to provide needed assistance in emergency situations.
- Targeted delivery – These injectables can be formulated to target certain cells, increasing efficacy and reducing the potential for systemic side effects.
- High bioavailability – Small molecule injectables often can display high bioavailability, meaning a portion of the injected dose reaches the target organs or tissues. This ultimately reduces the need for higher doses, leads to lower costs, and can produce fewer side effects.
- Flexible dosing – By using injectables, more control over the dosage is possible. This allows for treatments to be adjusted based on patient needs.
- Stability and shelf life – Small molecule pharmaceuticals usually have a longer shelf life compared to other complex drug formulations. This is important when it comes to the storage and distribution of drugs.
- Potential for self-administration – These injectables can provide greater independence for patients, as some can be designed for self-administration.
- Improved patience compliance – In the event that a patient has trouble taking oral medication, these small molecule injectables provide a great alternative.
How Small Molecule Pharmaceuticals Play a Key Role in Medicine
With their ability to target specific areas in the body and provide therapeutic benefits, small molecule injectables have become a vital part of drug development and can be used for a variety of roles, such as in:
- Pharmacokinetic studies
- Post-marketing surveillance
- Clinical trials
- Regulatory approval
- Preclinical studies
- Formulation development
- High-throughput screenings
- Structure-activity relationship (SAR) studies
- Lead compound identification
- Target identification or validation
Small molecules have helped transform a wide range of medical conditions, making them an essential tool in the drug development process.
Where Oakwood Labs Steps In
Oakwood Labs has been an effective partner for a variety of pharmaceutical firms of diverse sizes. We pride ourselves on providing transparent communication, achieving milestones within set deadlines, and offering our expertise throughout the entire drug development process.
We have over 20 years of experience in developing sustained-release injectable pharmaceutical products, including small molecule injectables. Our team can help you if you are in the early development stages or are looking to scale up an existing formulation.
With state-of-the-art technology and dedicated personnel, we can help you solve all of your challenges, and our facilities are adept at handling small molecule injection manufacturing and more.
Our cGMP manufacturing facility is FDA-approved and was created to enhance our partnering and development capabilities. Features of our facility include:
- Aseptic filling
- In-house QC microbiology and QC chemistry
- Inspecting, labeling, and packaging
- Aseptic formulation
- Compact and portable equipment train
- Non-aseptic formulation
- Raw material dispensing and weighing
- Vial capping
- Shipping, receiving and warehouse space
- Aseptic pharmaceutical lyophilization
By working with us, you can expect benefits such as short lead times, robust quality systems, and CMC development capabilities for a wide selection of products.
Contact Oakwood Labs About Small Molecule Injection Manufacturing
If you’re interested in learning more about small molecule injection manufacturing, contact our team today. We are committed to developing products that provide therapeutic benefits to patients and excellent returns to our partners.
Microspheres vs. Microcapsules: Key Takeaways
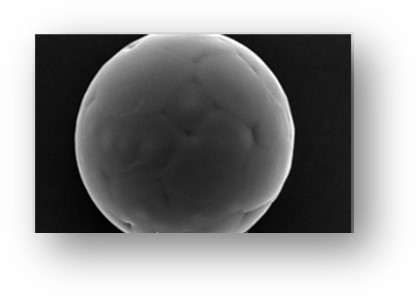
- Microspheres and microcapsules are both microparticles used in sustained-release injectable pharmaceuticals, but they have distinct structures. Microspheres are hollow with a solid outer casing, while microcapsules have a solid or liquid core enclosed in a shell-like coating.
- Microspheres, ranging from 1 μm to 1 mm in size, are made of polymers and can be delivered through various routes, including oral, parenteral, nasal, ophthalmic, and transdermal methods.
- Oakwood Labs has a patented microsphere technology, Chroniject™, which offers several advantages, including rapid formulation development using small-scale batches, easy scalability, compatibility with various molecules, and flexible release durations from one week to one year.
As you know, Oakwood Labs is a global leader in sustained release injectable pharmaceuticals. One of the key components of these drugs are microparticles, which are delivery systems for water-insoluble and sparingly water-soluble agents. Microparticles are split into microspheres and microcapsules.
Both of these play an important role in the pharmaceutical development process at Oakwood Labs. Learn more about microspheres vs. microcapsules.
What Are Microspheres?
Microspheres are small spherical particles that have a solid outer casing, are hollow, and do not contain fluid inside. These particles are made out of polymers and are used in drug delivery applications due to the high surface area, but low particle size. Their size ranges from 1 μm to 1 mm, making them effective for traveling within different areas throughout the body, which helps increase absorption rate and bioavailability.
Microspheres can be delivered through oral, parenteral, nasal, ophthalmic, and transdermal methods. They attach to proteins, peptides, antibodies, antigens and other small molecules to travel through the body and release at controlled rates.
Using microspheres in drug delivery has many benefits including release rates are tailored to specific applications (constant rate of delivery or rapid release), an increase in patient comfort and compliance by reducing the dosing frequency, and more.
What Are Microcapsules?
A microcapsule is also a small spherical particle, but its size is a little different, being 50 nm to 2 mm. The structure of a microcapsule is that of a membrane-enclosed core with a shell-like coating. Sometimes there are multiple cores within the coating. The core is composed of a drug constituent, stabilizers, or additives. The outside coating consists of an inert polymer, coloring agent, plasticizers, resins, waxes and lipids.
Microcapsules allow the core to be isolated from the outside environment and then released when desired. These microcapsules are used for sustained drug release, converting liquid drugs in a free-flowing powder, reducing toxicity and GI irritation, and for drugs that are sensitive to oxygen, moisture or light.
The Key Differences Between Microspheres and Microcapsules
The major difference between microspheres vs. microcapsules is the structure of the particle. As mentioned previously, microspheres have a solid casing and are hollow, while a microcapsule has a solid or liquid core in a shell-like coating.
The main reason for microencapsulation in pharmaceuticals is to protect drugs from environmental hazards like humidity, light, oxygen or heat. They also help controlled release within the patient and avoid too rapid of a release.
Microspheres are manufactured out of different types of polymers that when injected react within the patient to release a certain amount of the drug. While microspheres and microcapsules achieve similar goals for controlled drug release, their structures are what sets them apart.
Our Process for Manufacturing Microsphere Technology
At Oakwood Labs, our microsphere manufacturing capabilities have solved complex pharmaceutical industry challenges for more than 20 years. Our microsphere technology Chroniject™ is a patented, polymer-based injectable microsphere system for drug delivery. This technology is unmatched, and some of its major benefits include:
- Use of small-scale batches allow for rapidly developed formulations
- Formulations are easily scalable
- Process parameters are well-controlled
- Compatibility with peptides, small molecules, and proteins
- Excellent stability
- Flexible release durations ranging from one week to one year
Reach Out Today for More Information on Microcapsules and Microspheres
Our team is fully equipped and operates a staffed research and development facility specifically created to manufacture sustained release injectables. Oakwood Labs is committed to developing products to provide therapeutic benefits to patients.
If you are interested in learning more about microspheres vs. microcapsules for drug delivery, contact us at Oakwood Labs today.
Medications are classified and categorized in many ways including when it comes to distribution methods, dosage, intent, and more. For the purposes of this post, we are going to focus on the rate at which medications are released in the body to serve their intended function.
The time it takes for a drug to run its course will impact all of the characteristics mentioned above, so we’ll look at extended-release medication and modified-release medication and how it applies to drug delivery.
What Is the Difference Between Immediate-Release Drugs and Extended-Release Drugs?
Immediate-Release Drugs
For the purposes of comparison and to fully understand the role of extended-release medications, we’ll start by acknowledging the most common oral dosage for medication, which is immediate release. Any medications classified as immediate release, or IR, go to work very quickly and for a short period of time.
Modified-Release Drugs
As part of an effort to administer medications more conveniently and provide worthwhile options for patients, modified-release medications began appearing on the market. These medications were developed to extend and/or delay their effects to better aid the patient. Their design often means that a patient is required to take medication less frequently, which in many cases is majorly beneficial to their lifestyle.
Modified-release drugs, also known as extended-release drugs or slow-release drugs, are designed to last longer in your body. For example, someone administered an extended-release medication may only need to take one to two doses as opposed to three to four doses of an IR medication. From a physiological standpoint, these medications are absorbed much slower by the patient and do not start breaking down until they reach a certain area of the body.
What Purposes Do Slow-Release Drugs Serve and How Can Oakwood Labs Help?
As mentioned above, modified-release drugs require less frequent doses while still delivering the same medicinal benefits as their counterpart IR medications. This can be quite beneficial when it comes to an individual who has trouble taking medication on a set schedule or more than once a day.
One type of extend-release medication that is frequently used is long-acting injectables. Oakwood Labs is a leader in the development and manufacturing of these extended-release injectables, and some benefits of this type of drug administration include:
- Fewer injections needed over a determined period of time
- Ability to target precise areas of the body
- Improved patient compliance
- Help in preventing drug abuse
Our team has over 20 years of drug encapsulation experience and can work closely with you in developing long-acting injectables for your needs. Visit our homepage to learn about everything we can provide.
Contact Oakwood Labs for Extended-Release Medications
Oakwood Labs has been working in pharmaceutical development for over two decades and has a team of dedicated scientists ready to help your business from concept to finished product.
If you are interested in working with us or would like to learn more about slow-release medications, contact our team today!
Designed to work as a long, persistent, and rigorous process, drug development is crucial to progressing the field of medicine. Drug researchers and developers are tasked with expanding on previous information and findings in order to combat the rise of new, as well as prevalent, diseases.
Because of this, our team at Oakwood Labs wants to highlight the five drug development stages to further encapsulate the rigor of the process of providing sustained-released injectable pharmaceuticals for the community.
Drug Development Stages Broken Down
As addressed by the FDA, the stages of drug development follow a similar trend for each case. Because of this, we have outlined each drug development stage below, as we feel it is important for our clientele to have an inside look at the processes that we adhere to.
Discovery and Development
Known as the first stage of drug development, the discovery and development stage starts with an initial idea that is fabricated through consistent research and trends. During this stage, scientists discover new insights into the disease process, which allows them to develop a design for a product to either stop or reverse the effects of the disease under consideration. From there, several tests of the molecular compounds are tested to find the possible beneficial effects, or even test existing treatments that might possibly have unanticipated effects.
Once researchers find results of a promising compound through the discovery process, they conduct further experiments to gather information on the following hypotheses:
- How the promising compounds absorb, distribute, metabolize, and dispose of waste
- The mechanisms of action, and how they can be beneficial to further drug development
- The best dosage of the future drug
- The best way to give or take the drug, such as by mouth or as an injectable
- The side effects of the drugs, also known as toxicity
- How it affects individuals of different races, genders, and ethnicities differently
- How it correlates and interacts with other drugs and treatments
- Its overall effectiveness compared to similar treatments
After conducting the series of tests to gauge additional information, researchers are then ready to conduct the preclinical research stage of drug development.
Preclinical Research
As a precautionary measure, researchers must further test their drug for toxicity before distributing the finalized product for public use. Presented in the form of either in vitro or in vivo research, this stage of drug development must be conducted in favor of good laboratory practices (GLP), which can be found in 21 CFR Part 58.1: Good Laboratory Practice for Nonclinical Laboratory Studies. These regulations essentially set the minimum basic requirements for features, such as study conduct, personnel, facilities, equipment, written protocols, and operating procedures, among others.
Though preclinical studies are not usually large, they must provide very stringent and detailed information regarding dosing and overall toxicity levels. Once completed, researchers will assess their findings and decide whether the drug should proceed to the clinical research stage of drug development.
Clinical Research
During this drug development stage, the drug that was initially tested in the preclinical trial will now be tested on people with the hopes of finding how it will interact with the human body. But before the clinical trial can even start, developers will need to design the clinical study for the trial.
When designing a clinical trial, developers follow a specific study plan or protocol that is initially developed by the researcher or manufacturer. Then, the researchers will review the information from the previous two stages to develop research questions, objectives, and decide the following:
- A selection criterion of who qualifies to participate
- The number of people allotted for the study
- The duration of the study
- If there should be the presence of a control group to limit research bias
- How and at what dosage the drugs will be administered
- What assessments will be conducted and what data will be collected
- How the collected data will be reviewed and analyzed for future drug development stages
Unlike the preclinical trials, the clinical trials range from early, small-scale studies to late-stage, large-scale studies.
FDA Review
Considered the final drug development stage, the FDA review team will thoroughly examine the evidence from the drug’s early tests, preclinical and clinical research, and decide to either approve or not to approve the product.
During this stage, a New Drug Application (NDA) must be provided to outline the entirety of the drug development stages that lead to the anticipated approval. The NDA must include the following when presented:
- Proposed labeling
- Safety updates
- Drug abuse information
- Patent information
- Any data of studies that were not conducted within the United States
- Institutional review board compliance information
- Clear and concise directions for use
Finally, the drug is sent for approval to the FDA, where the committee decides whether the drug is safe and effective for public use. The process of approval can take anywhere from 6 to 10 months, as it goes through several inspectors, project managers, and pharmacologists.
From there, the drug is passed to the labeling and marketing stage, where the remaining issues will need to be resolved, and labeling will be provided accurately and objectively for public discretion.
FDA Post-Market Safety Monitoring
Though there are rigorous and persistent steps during each stage of drug development up to its approval and distribution, limitations are still evident and existent. Because of this, the FDA closely monitors drugs for months and even years so they can review the reports on the prescription and decide whether or not to add cautions to the dosage or usage information.
If there were significant changes that needed to be made to a product after the drug development stages were complete, developers would have to file a supplemental application in order to make those changes.
Why Choose Us for Your Pharmaceutical Needs
At Oakwood Labs, we are honored to provide sustained-released injectables to our extensive clientele. On top of that, some of the benefits of working with our pharmaceutical company through each stage of drug development include the following:
- Pre-formulation development
- Feasibility studies
- Scaling formulation
- Toxicology batch manufacturing
- ICH compliant stability tests
- Small-scale to large-scale clinical trial manufacturing
- Commercial and aseptic GMP manufacturing
Contact Oakwood Labs to Learn More About the Stages of Drug Development
If you have any specific questions regarding the drug development stages that Oakwood Labs conducts for sustained-release pharmaceuticals, please contact our team today. We look forward to assisting you in any way we can.