Feasibility Program
Feasibility Program
Oakwood has over 20 years of experience developing sustained-release injectable pharmaceutical products, including small molecules (both hydrophilic and hydrophobic), peptides, and proteins. We use a variety of polymers with proven safety profiles to develop formulations with in vivo durations of two weeks to one year. We work with clients to determine the optimal target product characteristics in terms of selecting the correct polymer, identifying the appropriate solvent system, tailoring the duration of release, and achieving optimal drug load and encapsulation efficiency.
Oakwood’s Feasibility Program
Starting a feasibility study is a quick way to determine if the target product profile is achievable with our technology. We pride ourselves in completing the feasibility study in a quick manner, within 3-4 months, with high-quality results. Our typical approach and activities related to feasibility are mentioned below.
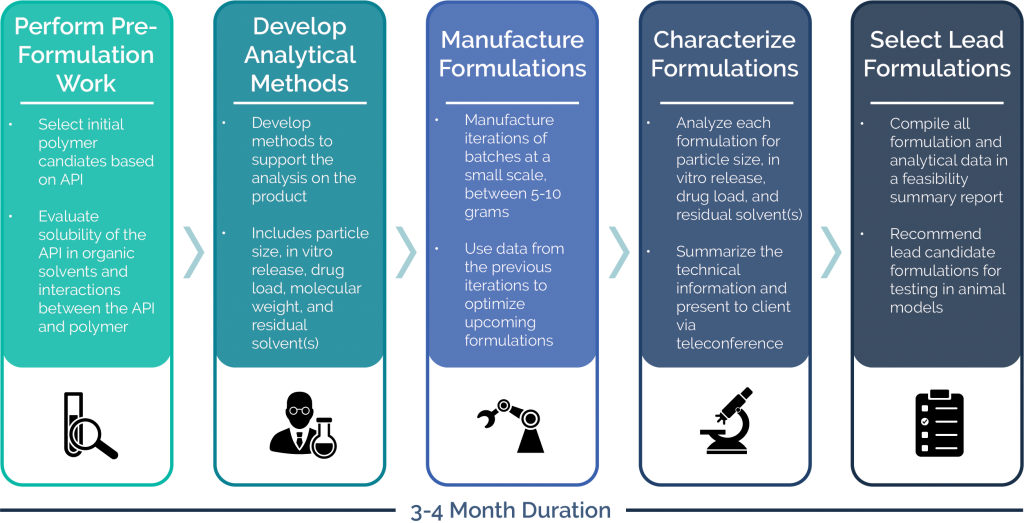
1. Perform Pre-Formulation Activities
Prior to manufacturing the feasibility batches, Oakwood will perform several pre-formulation activities. The results of these activities will form the methodology of how we develop a strategy for batch production.
- Select initial polymer candidates based on our expertise and internal database
- Evaluate solubility of the API in organic solvents and the interaction between the API and polymer
2. Develop Analytical Methods
Oakwood has an in-house analytical capabilities within our research and development team. In order to determine if the target product attributes are achievable, analytical methods must be developed.
- Transfer in existing client analytical methods
- Perform preliminary development of analytical methods to support the analysis of the product
- Typically analytical methods include particle size, in vitro release, drug load, residual solvent, and molecular weight
3. Manufacture Formulations
Oakwood manufactures feasibility batches at a small lab-scale, typically between 5-10 grams, in an iterative process of manufacturing and testing. Using the data obtained from the first batches, variation of polymer molecular weight, drug load, particle size, flow rates, and other factors that impact the release profile are tried in an iterative process.
- Present to the client proposed batches
- Manufacture microsphere formulation pilot batches utilizing Oakwood’s technology
- Vary formulation parameters for further batches as needed
4. Characterize Formulations
Similar to the iterative process of manufacturing, testing of all formulations made during feasibility occurs. Each formulation will be tested for drug load, particle size, residual solvents and in vitro release. Oakwood prides itself on transparent communication and all technical information is summarized and shared with the client. The characterization results will guide tweaks to the formulation to achieve the desired target product profile. A summary of analytical analysis and methods are shared below.
- Characterize microsphere formulation pilot batches
- Summarize the technical analysis and present results to the client via teleconference (typically bi-weekly)
| Analysis | Method |
| Drug Load (% drug encapsulated) | HPLC Assay |
| Particle Size and Volume Distribution | Laser Diffraction |
| Residual Solvent | Gas Chromatography |
| In Vitro Release | Shaker Bath or USP II/IV Apparatus, HPLC Assay |
5. Select Lead Formulations
Oakwood will compile all microsphere formulation data and analytical results to date in a feasibility summary report. In the report, we will recommend lead candidate formulations (usually three to five), based upon in vitro characterization results, for testing in animal models. Animal testing is typically conducted in dogs or rats and is outsourced. Note that in vitro release results are indicative, but are not necessarily predictive, of in vivo performance.
- Finalize feasibility summary report including all results to date
- Recommend lead formulations for animal study
- Supply material for initiating animal study
Next Steps for Microsphere Formulation Development
After completion of the feasibility study and animal testing, we can determine the predictive capability of the in vitro release test, and use the in vitro testing for further modification of the release profile if necessary. Additional animal testing may or may not be conducted. The microsphere formulation is then ready for initial scale-up.
If you have any additional questions about our microsphere formulation development process, or any of our other services, don’t hesitate to contact us below.

