Archives by Month: February 2024
Oakwood Labs is a global leader in sustained-release drug delivery. Our facility provides feasibility studies, analytical development, scale-up capabilities, GMP clinical trial material, GMP contract manufacturing, and sterile fill-finish.
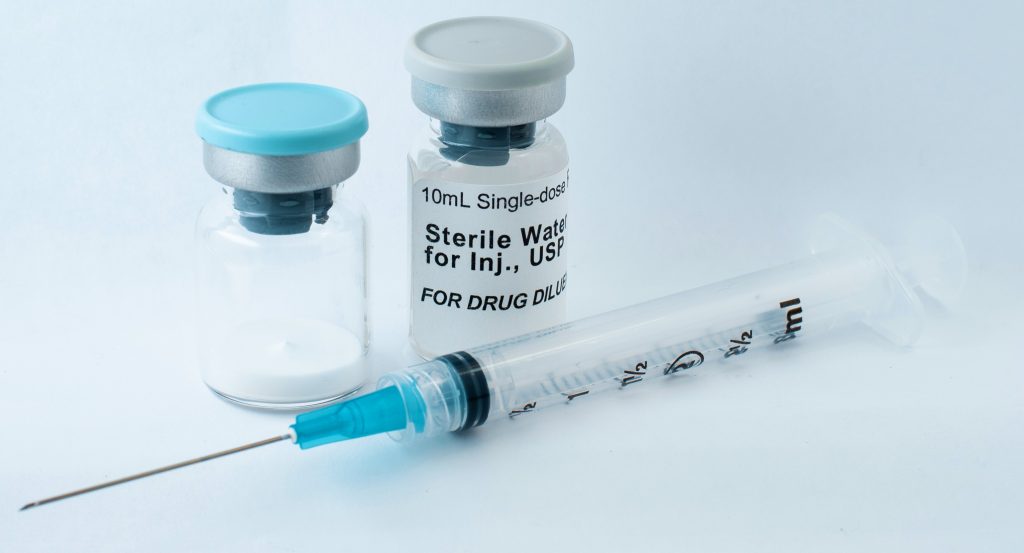
Sterile fill-finish is the final leg of the pharmaceutical manufacturing process, where a substance is filled into vials, ampoules, or other containers deemed appropriate. After the substance is put into containers, it is ready to be sent out for distribution. Learn more about our capabilities for this part of the process below.
The Process for Fill-Finish Pharmaceuticals
There are a variety of reasons that sterile fill-finish is essential in the manufacturing of long-acting injectables. Patient safety is at the top of the list, however, as the approach ensures that the product is free from microbial contamination.
What the Process Entails
When you begin the sterile fill-finish step, you are almost at the end of the drug manufacturing process. However, this is a critical point of the overall manufacturing process because it needs to be done correctly to ensure that your drug is safe for patients. At this point, you’ll want to request help from a fill-finish CDMO who can oversee the process properly.
Some of the steps that will be completed will include:
- Filling – In the filling process, the drug is placed into the final product containers under controlled and sterile conditions. Finish and fill services must be completed in a sterile environment to prevent contamination. For this reason, these services are performed in a cleanroom that has controlled air quality, temperature, and humidity. Additionally, it is important to ensure that sterile filling techniques are also applied to equipment and packaging.
- Container closure – It is important to select the appropriate container closer that is going to maintain the sterility and stability of the drug. Whether this is a vial, syringe, ampoule, or something else, the team you work with will help find the right solution for your application.
- Quality control – In the process of fill-finish for pharmaceuticals, rigorous quality control is practiced to ensure that the product meets regulations. Not only is the product tested throughout the process, but it also undergoes environmental monitoring and final product review to ensure that it is sterile and potent, and that it meets all other criteria that is required.
- Labeling and packaging – Once the product is filled, the finished item is labeled and packaged according to regulations and any requirements provided by outside sources.
- Regulation compliance check – It is important for aseptic, CDMO facilities to adhere to regulatory guidelines like Good Manufacturing Practice (GMP). This helps to ensure safety, efficacy, and quality, completing the final steps of the finish and fill process.
When you choose to work with a fill-finish CDMO, you can also receive different batch sizes to meet your project’s needs. From small-scale clinical trials to large-scale commercial production, our processes can be adapted to your specifications.
Let Oakwood Labs Be Your Fill-Finish CDMO
Oakwood Labs operates a fully compliant aseptic GMP facility. Our FDA-approved facility is headquartered near Cleveland, Ohio and centers on the development and manufacturing of sustained-release injectables for the pharmaceutical industry. We focus on creating pharmaceutical products that provide therapeutic benefits to our patients and excellent returns for our partners.
Whether you need assistance with fill-finish pharmaceutical services or something else, we are here to help. Other services we provide include:
- Formulation and analytical development activities
- Accelerated and long-term stability studies
- Scale-up, validation, or engineering batches
- Complete method transfer and validation
- Batch record and protocol development
Consult Our Finish and Fill Services Today!
If you are in need of pharmaceutical manufacturing, partner with Oakwood Labs to receive reliable and efficient services.
Contact us today to learn how you can achieve a sterile fill-finish with our CDMO.
Best known for their versatile role in a vast array of chemical applications, organic solvents play a crucial role in numerous sectors, including the medical industry.
To gain a better understanding of how these solvents can contribute to pharmaceutical practices and manufacturing techniques for drug delivery, review our complete guide for more information.
An Overview of Organic Solvents
As industry experts are aware, organic solvents are solvents that contain carbon atoms capable of either dispersing or dissolving one or more substances. Due to their chemical makeup, these solvents can be utilized in a variety of industries, such as textiles, cleaning, agriculture, and pharmaceuticals.
Additionally, common characteristics and properties of suitable organic solvents include the following:
- High volatility
- Acid-based properties
- Low boiling point
- Suitable density
As opposed to some inorganic solvents, organic solvents can also be described as combustible and extremely flammable. That’s why we advise that those looking to work with organic solvents in long-acting injectables, or processes such as spinning and dyeing textiles, ensure that they are handling these carbon-based solvents with the utmost care.
The Role of Organic Solvents in Long-Acting Injectables
Organic solvents in pharmaceuticals, such as long-lasting injectables, can contribute to an expansive array of roles throughout the drug delivery process, such as the following:
- Dissolving drugs at high concentrations – Organic solvents can be utilized to dissolve drugs, such as biologics, that otherwise have a higher concentration and a lower aqueous solubility. That way, long-acting injectables can be administered at the appropriate viscosity and form drug depots under the skin’s surface.
- Forming drug depots – Drug depots, or depot injections, are longer-duration drugs that result in a slow absorption into the bloodstream. Though organic solvents can contribute to the formation of these drug depots, the administration of the drug into your muscle is best when using a depot.
- Determining the rate of release – Once the drug depot has formed, organic solvents decelerate the rate of their delivery, resulting in a more gradual, sustainable release and absorption of the medication over time.
A Look at Organic Solvents in Pharmaceuticals: Their Industry Uses and Beyond
Along with their inherent contribution to the production of long-lasting injectables, organic solvents in pharmaceuticals play many key roles in the total industry. Beyond mainly being utilized as reaction media, organic solvents are used in the following processes:
- The purification of synthesis products
- Excipients, such as coloring agents, preservatives, and fillers
- The bioavailability of orally administered protein-active sites
- Semiempirical models of meditated solubility
- Antisolvent crystallization techniques in pharmaceutics
Additionally, organic solvents can even be used in water-based cleaning solutions. This is because they are capable of penetrating oily soil deposits while also dissolving in water.
FAQs About Organic Solvents, Their Capabilities, and More
What are the most common organic solvents used in pharmaceutical manufacturing?
The most common applications when working with organic solvents in pharmaceuticals include the following:
- Acetone
- Dimethylformamide
- Diethyl ether
- Benzene
- Isopropanol
- Ethanol
What safety precautions should be taken when working with organic solvents in pharmaceuticals?
As stated previously, organic solvents can be extremely flammable and volatile, so handling them with care is essential. With that in mind, here are some stringent safety regulations you should abide by during the manufacturing process:
- Gloves and safety glasses should be worn when handling the materials
- A fume-hood is required when pouring out organic solvents
- Spill kits and eyewash stations should be readily available in the workspace
- Flammable liquids, such as bleach, peroxides, and acids, should be stored away from organic solvents
Can organic solvents be reused or recycled?
Yes, organic solvents can be recycled easily and reused in syntheses. This includes collected solvents that are separated by distillation and have different boiling points.
Contact Oakwood Labs for More Information
As your trusted resource for sustained-release injectables and other services, Oakwood Labs is well-equipped with the necessary tools to satisfy your application needs.
For more information about organic solvents in pharmaceuticals, long-acting injectables, and more, please reach out to our team today. We look forward to assisting you.