Global Leader in Sustained Release Drug Delivery
Oakwood specializes in a full service offering of end-to-end solutions to support all phases of long acting injectable (LAI) development. As an experienced global CDMO partner focused on quality and timeline driven development, we will work with you from initial feasibility assessment, in vivo confirmation studies, GLP toxicology, product stability studies, scale-up and optimization, technology transfer to our GMP facility, and aseptic manufacturing of clinical and FDA-approved commercial supply.
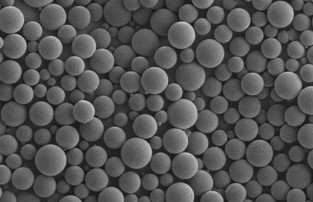
Oakwood has over 25 years of drug encapsulation experience using Chroniject™, our in-house patented microsphere-based technology. The technology has allows the ability to control particle size distribution, customize and tailor release durations from weeks to years, and minimize residual solvents to achieve critical quality attributes. Chroniject™ is advantageous due to its scalability. We have proven the ability to translate lab-scale formulations into multi-kilogram validated processes.
The Chroniject™ platform is compatible with a variety of molecule types including small molecules, peptides, and proteins. Our long-acting injectable technology has applications within various therapeutic markets including Central Nervous System Disorders, Neuroscience, Ophthalmology, Oncology, Cardiovascular, Depressive Disorders, and Endocrinology.
Schedule an Introductory Call Today
Formulation Development
Ability to encapsulate various molecules in a microsphere, achieving release durations ranging from weeks to one year.
Scale-Up and Tech Transfer
Proven scalability starting from lab scale all the way to commercial batch sizes, as well as lot-to-lot reproducibility.
GMP Aseptic Manufacturing
FDA approved aseptic manufacturing facility to produce and package supply of clinical and commercial material.
Why Choose Oakwood for Sustained Release Pharmaceuticals?
At Oakwood Labs, we know that developing a sustained release drug from concept to commercialization requires a successful partnership. Over the last 20 years, we have proven ourselves as an effective partner to global pharmaceutical firms of various sizes. We pride ourselves on our ability to achieve milestones within set deadlines, provide transparent communication, adapt to change, and offer our expertise throughout the process.
Experts in Sustained Release Drug Delivery
Oakwood Labs Overview
Based in the Cleveland, Ohio area since 1997, Oakwood Labs is a leader in the pharmaceutical industry offering contract development and manufacturing services. With state-of-the-art technology, personnel, equipment, and facilities, our company specializes in the development, scale-up, and aseptic manufacturing of sustained-release injectable microsphere-based formulations. Our technology solves all of the challenges involved with developing and manufacturing pharmaceutical injectables. These challenges include:
- Formulation development
- Scaling up and process controls
- GMP manufacturing under rigorous in-house quality systems
We also offer contract manufacturing services for sterile liquid and lyophilized products. All commercial products and sustained release pharmaceuticals are manufactured in our FDA-approved, aseptic GMP facility.
Injectable Microsphere Development
Our microsphere technology, Chroniject™, is a patented polymer-based injectable microsphere system for sustained release drug delivery. This technology is compatible with small molecules, hormones, peptides, and proteins. Our ability to demonstrate lot-to-lot reproducibility and scale-up success is what sets us apart from other manufacturers of sustained release drugs. Over our 20-year history, we have scaled up numerous client projects from initial feasibility to production of multiple Phase 3 clinical batches.
At Oakwood Labs, we typically start with initiating a feasibility study that utilizes small batch sizes to enable rapid formulation development and testing of numerous trial batches to obtain a formulation with the desired release profile. In parallel, we develop analytical methods to test prototype formulations of the sustained release drug delivery.
Once several promising formulations are tested in vitro, animal studies are performed to determine if the in vitro testing is reflective of the in vivo release profile and to select a formulation to scale up for human testing. A confirmatory animal study may be conducted with the scaled-up material manufactured in our GMP facility. Our GMP facility can produce all clinical trial material and commercial supplies when needed. The entire process is carried out under full and robust quality systems.
Looking to Partner with a Leading Sustained Release Drug Manufacturer? Reach Out to Our Team Today!
Oakwood Labs is a “one-stop shop” for developing complex microsphere products and sustained release drugs from concept to commercial supply of a product. Whether you are early on in development or looking to scale up an existing formulation, we are here to help.
We pride ourselves on being highly transparent and user-friendly with our clients. All work is carried out under project plans agreed to by our customers beforehand. Progress reports and review meetings are provided as frequently as desired.
Our specialty pharmaceutical company has the experience to meet your requirements and expectations for microsphere-based sustained release pharmaceutical injectables. Contact us today to explore further how Oakwood Labs may provide solutions to your company.