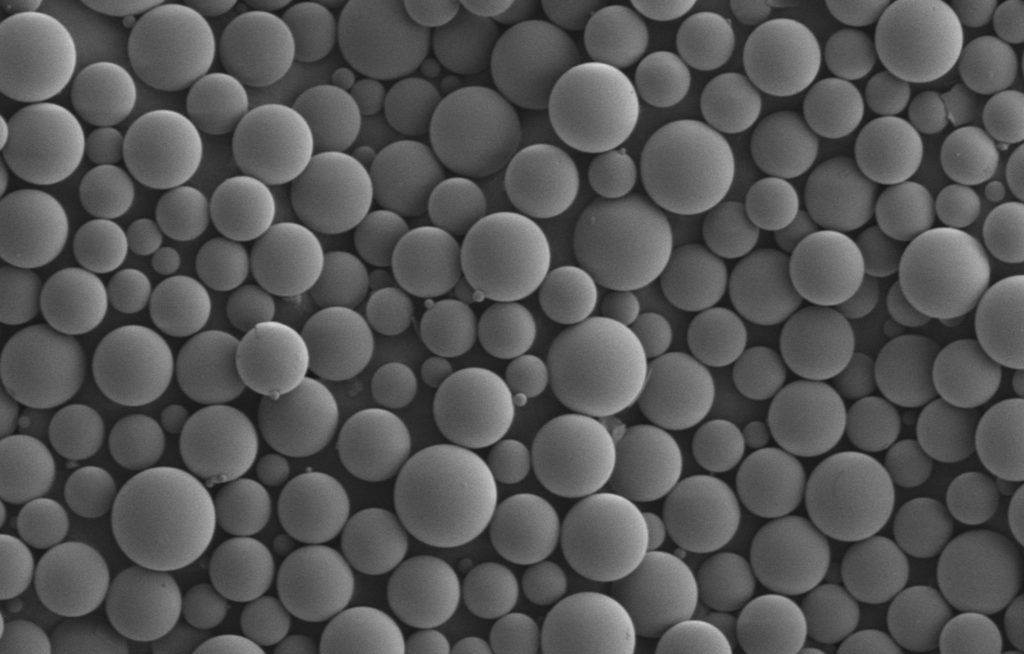
Author Archives: Mark Ilhan
The medical field is always searching for new opportunities to help treat patients, and one technique that provides various advantages is drug repositioning. This innovative technique involves exploring new medical uses for existing drugs.
At Oakwood Labs, we are a leader in the pharmaceutical industry, solving difficult challenges that arise from developing pharmaceutical injectables. Similar to the goals of drug repurposing, we strive to develop products that provide therapeutic benefits to patients and practical benefits to medical operations.
Utilizing Repurposed Drugs for Cancer
Focusing specifically on oncology and the effort to find new treatments, repurposed drugs for cancer have become an attractive option, with some non-cancer drugs being examined for their possible efficacy. Many unrelated diseases share common molecular traits, making repurposing possible. It also opens the door for different diseases to respond to the same drug.
Traditionally, bringing a new drug on the market would take 12 to 15 years and would require a large budget of 1 to 3 billion dollars. By choosing the approach of drug repositioning, you’ll reap benefits such as:
- Cutting costs surrounding research and development
- Reducing the timeline of development, because if the drug has already been safely administered to humans, you could skip Phase 1 of clinical trials
- Higher approval rates
- Comprehensive information is available on pharmacology dose, possible toxicity, and more
The Role of Drug Repositioning in the Greater Pharmaceutical Industry
Pharmaceutical companies continue to evolve, shaping the future of drug development by finding innovative solutions, such as drug repurposing. With many rare diseases in the world having few approved treatment options, repositioning of drugs has become an effective strategy.
Utilizing this strategy for such cases, facilities can minimize the knowledge gap that occurs when running a clinical trial. By understanding more about the drug, such as how it reacts in the body, organizations can also help speed up the process.
Since drugs are chemicals that change the biochemical pathways in our cells, it’s common for them to have an effect on more than one pathway. In drug repositioning, we can take advantage of those different effects to provide solutions to diverse medical conditions.
Oakwood Labs, a Leading Manufacturer in the Industry
With over twenty years of industry expertise, we have proven ourselves as an effective partner to global pharmaceutical firms of various sizes. Whether you are in the early development stage or are looking to scale up an existing formulation, we are ready to help.
Oakwood Labs strives to achieve milestones within set deadlines, provide transparent communication, adapt to change, and offer our expertise throughout the entire development process. We have everything you need, from support to our FDA-approved cGMP manufacturing facility, all to help enhance our development capabilities.
Our facility offers a variety of contract manufacturing services, including:
- Commercial products for use in humans or for veterinary use
- Phase I, Phase II, or Phase III clinical trial material
- Sterile liquids, sterile lyophilized parenteral, suspensions, microspheres, and others
- Small-volume injectables
- Class III and IV DEA controlled substances
- Vials with lyophilized or plug stoppers, with both 13 mm and 20 mm openings
Contact Our Team
At Oakwood Labs, we are ready to use our technology and expertise to tailor solutions to your project’s needs. If you’re interested in drug repurposing and would like to discuss options with us, contact our team today.
We are ready to support all phases of long-acting injectable development and help you with your next project.
Sustained-release injectables provide a solution for those that require long-term treatment, or in cases where patients can’t swallow tablets or capsules. Also, depending on the drug, certain tablets cannot be crushed, making injectables the preferred option as well.
Oakwood Labs focuses on long-acting injectable development since these medication delivery methods can reduce the number of injections needed, target specific anatomical areas, and help improve patient compliance. By choosing injectables over capsules, you’ll achieve the quality and efficacy you’re seeking.
What Are Sustained-Release Tablets?
Disadvantages of Choosing a Tablet Over an Injectable
While they do offer some benefits, there are distinct disadvantages to utilizing a sustained-release tablet instead of an injectable. Tablets have a higher risk of dosage issues, often known as dose dumping. This is when large amounts of a drug are quickly released, and a toxic quantity is introduced into systemic circulation. These capsules are also often quite large in size, which may cause issues in ingestion or transit through the digestive system.
Why Sustained-Release Injectables Are Beneficial
Sustained-release injectables are often the preferred method among clinical professionals due to the convenience and effectiveness they offer. While there are various types of injectables, depending on the medication they are utilized for the effects can last anywhere from two to twelve weeks with one dose. This provides a practical option for those having trouble taking medication on a daily basis.
By choosing injectables in place of sustained-release capsules, you will help improve the lives and routines of patients in the following ways:
- Safety – When administered by medical personnel, sustained-release injectables can help guarantee safety for your patients. Through regulated injections, you reduce the risk of accidental or deliberate overdose.
- Regularity – Patients who receive injectables need to show up for more appointments, creating regular interactions between them and their medical team. This results in a solid routine and an increase in social interactions.
- Stability – Sustained-release injectables help by treating patients with more stable plasma concentrations compared to oral medications. It also lessens the frequency of having peak plasma levels, which can result in lesser side effects for the patient. Also, for those prone to hospital visits, relapse frequency and rehospitalization rates are decreased.
- Reduced accountability – In order to see the best results with medications, it’s important for patients to take them every day. With long acting injectables, patients no longer need to keep track of daily dosing needs.
Choose Oakwood Labs as Your Source for Sustained-Release Pharmaceuticals
At Oakwood Labs, we understand how essential a partnership is when developing a sustained-release drug from concept to commercialization. With our state-of-the-art technology and personnel, we’ve been able to solve common challenges involved with developing sustained-release injectables such as formulation development, GMB manufacturing under rigorous quality systems, scaling up, and process controls.
As a specialty pharmaceutical company, we are here to help in development as well as scaling up an existing formulation. We support all phases of long-acting injectable (LAI) development, working with you through proof of concept to clinical trial material, all the way up to FDA-approved commercial supply.
By choosing Oakwood Labs as your effective partner, you’ll gain services and benefits such as:
- A strong focus on project management and communication
- Tailored release profile from weeks to one year
- Contract manufacturing services
- ChronijectTM, our patented injectable microsphere technology
- A track record of success for taking projects through every process stage
- Efficient and stringent process controls
- R&D formulation development
- Demonstrated lot-to-lot reproducibility with proven scale-up success
Contact Us to Learn More About Sustained-Release Injectables
Our special pharmaceutical company is ready to help ensure you meet your requirements and expectations for sustained-release injectables. If you’re wanting to learn more about how Oakwood Labs can provide solutions to your company, reach out to our team today.
Additional Resources on Sustained-Release Injectable Development
Medications are classified and categorized in many ways including when it comes to distribution methods, dosage, intent, and more. For the purposes of this post, we are going to focus on the rate at which medications are released in the body to serve their intended function.
The time it takes for a drug to run its course will impact all of the characteristics mentioned above, so we’ll look at extended-release medication and modified-release medication and how it applies to drug delivery.
What Is the Difference Between Immediate-Release Drugs and Extended-Release Drugs?
Immediate-Release Drugs
For the purposes of comparison and to fully understand the role of extended-release medications, we’ll start by acknowledging the most common oral dosage for medication, which is immediate release. Any medications classified as immediate release, or IR, go to work very quickly and for a short period of time.
Modified-Release Drugs
As part of an effort to administer medications more conveniently and provide worthwhile options for patients, modified-release medications began appearing on the market. These medications were developed to extend and/or delay their effects to better aid the patient. Their design often means that a patient is required to take medication less frequently, which in many cases is majorly beneficial to their lifestyle.
Modified-release drugs, also known as extended-release drugs or slow-release drugs, are designed to last longer in your body. For example, someone administered an extended-release medication may only need to take one to two doses as opposed to three to four doses of an IR medication. From a physiological standpoint, these medications are absorbed much slower by the patient and do not start breaking down until they reach a certain area of the body.
What Purposes Do Slow-Release Drugs Serve and How Can Oakwood Labs Help?
As mentioned above, modified-release drugs require less frequent doses while still delivering the same medicinal benefits as their counterpart IR medications. This can be quite beneficial when it comes to an individual who has trouble taking medication on a set schedule or more than once a day.
One type of extend-release medication that is frequently used is long-acting injectables. Oakwood Labs is a leader in the development and manufacturing of these extended-release injectables, and some benefits of this type of drug administration include:
- Fewer injections needed over a determined period of time
- Ability to target precise areas of the body
- Improved patient compliance
- Help in preventing drug abuse
Our team has over 20 years of drug encapsulation experience and can work closely with you in developing long-acting injectables for your needs. Visit our homepage to learn about everything we can provide.
Contact Oakwood Labs for Extended-Release Medications
Oakwood Labs has been working in pharmaceutical development for over two decades and has a team of dedicated scientists ready to help your business from concept to finished product.
If you are interested in working with us or would like to learn more about slow-release medications, contact our team today!
It is important to note that “extended-release pharmaceuticals” is an umbrella term for both sustained- and controlled-release pharmaceuticals. These drugs are part of a series called modified-release dosage forms, which refers to the medication’s time-release technology.
Typically, the drugs you ingest are immediate release, which means they are instantly activated when they are ingested. However, extended-release, sustained-release, and controlled-release drugs are modified pharmaceuticals designed to release at a delayed or slower rate to change how and when the drug hits your system.
A Breakdown on Extended-Release Pharmaceuticals
Extended-release drugs were created to offset the side effects that come with traditional, immediate-release drugs. IR drugs are designed to release a spike of medication into the bloodstream that then tapers off. Because of this, extended-release pharmaceuticals offset that spike of medication to provide a steadier amount of medication into the body.
Controlled-Release Medication
- These are medications that are labeled with “CR” at the end of their medical names
- CR drugs can release active ingredients at a very specific rate to ensure there is a constant medication flow in the body for a specific period of time
- Due to their medication-regulating abilities, fewer doses are required throughout the day
Sustained-Release Medication
- These are medications that are labeled with “SR” at the end of their medical names
- SR tablets or capsules prolong the medication’s release to dole out the benefits of the medication over a longer time period
- Although the medication is released over a longer period, the medication levels do not remain constant while in the body
Extended-Release Injectables from Oakwood Labs
Here at Oakwood Labs, we are your source for extended-release injectables. With over 20 years of drug encapsulation experience, we have patented technology to control particle size, customize release durations, and minimize residual solvents.
Our team of professionals has the ability to perform research and develop specially formulated extended-release injectables for our customers’ specific needs. Some benefits of our extended-release injectables include:
- Reducing the number of injections needed
- Ability to target specific anatomical areas
- Improved patient compliance
- Helping to prevent drug abuse
Extended-release drugs make it simpler for individuals to take medications on a regular basis, as these drugs are easier for the body to tolerate in comparison to immediate-release pharmaceuticals. Due to their modified composition, fewer doses are required, making it simpler to stick to a medication schedule.
Overall, extended-release pharmaceuticals allow your body to feel the effects of the specific medication over a longer time period, but via fewer doses. Understanding the specifics of extended-release drugs is important so you can predict how your body will react.
Contact Us for Extended-Release Drugs
Oakwood Labs is the best source for long-acting injectable manufacturing. Not only are we an industry leader in injectable pharmaceutical development, but we are very committed to quality and customer satisfaction.
If you have questions on extended-release injectables, contact us today for additional information!
Related Blog Posts

Applications for Microparticles in the Pharmaceutical Industry
Microparticles are generally made up of proteins or synthetic polymeric materials. They can be found in sizes ranging from 1 to 1000 mm, making them ideal for many different applications, including vaccines and other treatments. The following applications describe how they ease patients in need of relief and help the industry:
- When found in small sizes, microparticles are used for inhalation delivery because they are easily dispersed and aerodynamic, allowing them to travel to the deeper regions of the lungs.
- In addition to their role in transferring treatment to deep regions to the body, microparticles play an important role in the treatment and diagnosis of various cancers, including brain cancer, lung cancer, colon cancer, breast cancer, and ovarian cancer.
- Microparticles used in drug delivery have also shown promising results in reducing the treatment time of tuberculosis since they enhance the efficacy of the antibiotics delivered.
Reasons to Use Microparticles in Drug Delivery
Since they were first introduced to the medical field, microparticles have improved the efficiency of different medical treatments. The following advantages describe the benefits of using microparticles in drug delivery:
- Protective coating – Using microparticles supplies bioactive drugs, proteins, and small molecules with protection from degradation. This allows the microparticles to travel freely to their intended destination.
- Controlled release – Microparticles have the ability to effectively achieve a controlled release rate of encapsulated drugs, whether over a period of hours, weeks, or months. This can help in treating patients who forget to take their medication or those who do not want to attend as many appointments.
- Effective for various applications – Microparticles are effective because they can be used for a variety of drugs and can therefore be used in a variety of treatments. For example, they help cancer patients by reducing the number of chemotherapeutic drugs used in patients, reducing the side effects that the patient would otherwise experience.
How Oakwood Labs Can Help in Developing Effective Delivery of Microparticles
At Oakwood Labs, we specialize in formulating microparticles for pharmaceuticals. From the duration of release to the needle size, we work to determine the optimal product characteristics for your project. Other factors that we consider for your project include the following:
- Active pharmaceutical ingredient (API)
- Particle size
- Vial size
- Appropriate solvent system
- Selection of polymer
- Stable state levels
- Method of administration
- Drug load
Our Process for Incorporating Microparticles in Drug Delivery
After we determine the proper characteristics for your microparticle drug delivery, we begin the process of a feasibility study which will determine if the target profile is attainable with our technology. This process includes the following:
- Pre-formulation activities – Pre-formulation activities are used to develop the best approach for the production of microparticles.
- Completing feasibility formulations – The next step is to complete feasibility batches on a small scale. This data is later used to determine the microparticle weight, size, flow rate, and other factors.
- Finalizing reports and choosing formulations for testing – Our team will compile all of the appropriate data and results to form a summary of the microparticle After this, we will provide recommendations for candidate formulations that will be tested on animals. For the use of microparticles in pharmaceuticals and beyond, animal testing is outsourced.
- Further modification and scaling up – After animal testing, we determine the capability of the in vitro release test and use this in situations where further modification is necessary. At this point, the microparticle drug delivery system is ready to begin the process of scaling up.
Start the Process of Using Microparticles in Drug Delivery Today
When you are ready to include microparticles in your pharmaceuticals, be sure to work with a team that carries over 20 years of experience working in an aseptic, FDA-approved manufacturing facility.
Contact us today to learn more.
Designed to work as a long, persistent, and rigorous process, drug development is crucial to progressing the field of medicine. Drug researchers and developers are tasked with expanding on previous information and findings in order to combat the rise of new, as well as prevalent, diseases.
Because of this, our team at Oakwood Labs wants to highlight the five drug development stages to further encapsulate the rigor of the process of providing sustained-released injectable pharmaceuticals for the community.
Drug Development Stages Broken Down
As addressed by the FDA, the stages of drug development follow a similar trend for each case. Because of this, we have outlined each drug development stage below, as we feel it is important for our clientele to have an inside look at the processes that we adhere to.
Discovery and Development
Known as the first stage of drug development, the discovery and development stage starts with an initial idea that is fabricated through consistent research and trends. During this stage, scientists discover new insights into the disease process, which allows them to develop a design for a product to either stop or reverse the effects of the disease under consideration. From there, several tests of the molecular compounds are tested to find the possible beneficial effects, or even test existing treatments that might possibly have unanticipated effects.
Once researchers find results of a promising compound through the discovery process, they conduct further experiments to gather information on the following hypotheses:
- How the promising compounds absorb, distribute, metabolize, and dispose of waste
- The mechanisms of action, and how they can be beneficial to further drug development
- The best dosage of the future drug
- The best way to give or take the drug, such as by mouth or as an injectable
- The side effects of the drugs, also known as toxicity
- How it affects individuals of different races, genders, and ethnicities differently
- How it correlates and interacts with other drugs and treatments
- Its overall effectiveness compared to similar treatments
After conducting the series of tests to gauge additional information, researchers are then ready to conduct the preclinical research stage of drug development.
Preclinical Research
As a precautionary measure, researchers must further test their drug for toxicity before distributing the finalized product for public use. Presented in the form of either in vitro or in vivo research, this stage of drug development must be conducted in favor of good laboratory practices (GLP), which can be found in 21 CFR Part 58.1: Good Laboratory Practice for Nonclinical Laboratory Studies. These regulations essentially set the minimum basic requirements for features, such as study conduct, personnel, facilities, equipment, written protocols, and operating procedures, among others.
Though preclinical studies are not usually large, they must provide very stringent and detailed information regarding dosing and overall toxicity levels. Once completed, researchers will assess their findings and decide whether the drug should proceed to the clinical research stage of drug development.
Clinical Research
During this drug development stage, the drug that was initially tested in the preclinical trial will now be tested on people with the hopes of finding how it will interact with the human body. But before the clinical trial can even start, developers will need to design the clinical study for the trial.
When designing a clinical trial, developers follow a specific study plan or protocol that is initially developed by the researcher or manufacturer. Then, the researchers will review the information from the previous two stages to develop research questions, objectives, and decide the following:
- A selection criterion of who qualifies to participate
- The number of people allotted for the study
- The duration of the study
- If there should be the presence of a control group to limit research bias
- How and at what dosage the drugs will be administered
- What assessments will be conducted and what data will be collected
- How the collected data will be reviewed and analyzed for future drug development stages
Unlike the preclinical trials, the clinical trials range from early, small-scale studies to late-stage, large-scale studies.
FDA Review
Considered the final drug development stage, the FDA review team will thoroughly examine the evidence from the drug’s early tests, preclinical and clinical research, and decide to either approve or not to approve the product.
During this stage, a New Drug Application (NDA) must be provided to outline the entirety of the drug development stages that lead to the anticipated approval. The NDA must include the following when presented:
- Proposed labeling
- Safety updates
- Drug abuse information
- Patent information
- Any data of studies that were not conducted within the United States
- Institutional review board compliance information
- Clear and concise directions for use
Finally, the drug is sent for approval to the FDA, where the committee decides whether the drug is safe and effective for public use. The process of approval can take anywhere from 6 to 10 months, as it goes through several inspectors, project managers, and pharmacologists.
From there, the drug is passed to the labeling and marketing stage, where the remaining issues will need to be resolved, and labeling will be provided accurately and objectively for public discretion.
FDA Post-Market Safety Monitoring
Though there are rigorous and persistent steps during each stage of drug development up to its approval and distribution, limitations are still evident and existent. Because of this, the FDA closely monitors drugs for months and even years so they can review the reports on the prescription and decide whether or not to add cautions to the dosage or usage information.
If there were significant changes that needed to be made to a product after the drug development stages were complete, developers would have to file a supplemental application in order to make those changes.
Why Choose Us for Your Pharmaceutical Needs
At Oakwood Labs, we are honored to provide sustained-released injectables to our extensive clientele. On top of that, some of the benefits of working with our pharmaceutical company through each stage of drug development include the following:
- Pre-formulation development
- Feasibility studies
- Scaling formulation
- Toxicology batch manufacturing
- ICH compliant stability tests
- Small-scale to large-scale clinical trial manufacturing
- Commercial and aseptic GMP manufacturing
Contact Oakwood Labs to Learn More About the Stages of Drug Development
If you have any specific questions regarding the drug development stages that Oakwood Labs conducts for sustained-release pharmaceuticals, please contact our team today. We look forward to assisting you in any way we can.
In order for injectables to be able to successfully provide sustained releases, they necessitate drug-encapsulating devices. For over 50 years, encapsulation has been one of the many techniques used in the pharmaceutical industry. In this process, medication is released from a microsphere by the drug detaching from the polymer or through the degradation of the polymer matrix.
Keep reading to learn more about the role of polymer microspheres in the process of drug delivery and how our technology can be an asset to your long-acting injectable project.
The Use of Polymer Microspheres in the Pharmaceutical Industry
Polymer microspheres are often used in drug delivery because they have a high surface area and low particle size, which increases their absorption rate and bioavailability. Since these microspheres are small in size, they are also efficient when moving throughout the body. They are additionally capable of sustained release, making them ideal for use in long-acting injectables for both passive and active targeting.
Capabilities of Microspheres
Microspheres can be organic or inorganic and are designed to encapsulate bioactive molecules and release them in a controlled way. They are used for desired release profiles and can be used to target specific delivery sites throughout the body, including organs.
Additionally, they are considered rigid which means that they can be packed together, found alone, or even combined with other biomaterials to create porous 3D-structured scaffolds. In these scaffolds, they can either serve as a component of a larger scaffold or be the building blocks of one.
Factoring in the Polymer
Polymers can take the role of bioactive or biodegradable depending on the agents they are combined with. When incorporated with therapeutics, polymers become bioactive and provide their own therapeutic benefit. However, they can also be biodegradable which improves release kinetics and helps prevent carrier accumulation.
The benefits of using polymers for encapsulation include the following:
- Ability to encapsulate a variety of drugs
- They have high biocompatibility
- They are bioavailable
Incorporating ChronijectTM Technology into Your Project
Since polymer microspheres are suited for sustained-release technology, they are the perfect candidate for long-acting injectables. Our technology for microspheres is a patented, polymer-based injectable for drug delivery called ChronijectTM. This technology is compatible with small molecules, peptides, and proteins. It is also capable of enabling sustained release over durations of two weeks to one year.
Our ChronijectTM process for microsphere manufacturing initially uses small batch sizes to enable the production of numerous trial batches. This allows us to obtain a formation with the desired release profile and other indicated product characteristics. Additionally, this process can easily be scaled up to commercial production levels with lot-to-lot reproducibility. Since we can produce clinical trial material at the forecast commercial scale, scaling up is feasible at the commercial scale and is conducted in our GMP facility.
Advantages of Using ChronijectTM
There are diverse advantages of using polymer microspheres and our ChronijectTM technology for drug delivery, which include the following:
- Rapid development of formulations using small-scale batches
- Easily scaled formations
- Process parameters that are monitored and controlled
- Proven lot-to-lot reproducibility
- Molecule compatibility (small molecules, peptides, proteins)
- Flexible release durations, ranging from one week to one year
- Excellent stability
- Instant reconstitution with WFI – no special diluent required
- Applications in various therapeutic indications (CNS, neurology, ophthalmology)
About Oakwood Labs
Oakwood Labs is a specialty pharmaceutical company located near Cleveland, Ohio. We are focused on developing and manufacturing sustained-release injectable pharmaceuticals, incorporating our microsphere-based technology. We operate in a fully compliant, aseptic facility that is specifically used for manufacturing pharmaceutical products like injectables.
Our goal is to provide enhanced therapeutic benefits to patients while achieving excellent returns for our pharmaceutical partners. With over 20 years of experience, we have proven ourselves as an effective partner of global pharmaceutical firms. From proof of concept and feasibility studies to clinical trials and FDA-approved supplies, the team at Oakwood Labs is ready to help incorporate polymer microspheres into the development of long-acting injectables.
Contact Our Team with Any Questions
If you are interested in learning more about our capabilities in incorporating polymer microspheres into long-acting injectables, please reach out to our team today.
What Are Peptides?
Peptides are strings of amino acids, also known as the “building blocks” of proteins. The human body naturally produces more than 7,000 known peptide types. Along with the peptides that are naturally produced by the human body, they can also be produced in medical laboratories. Pharmaceutical peptides are those that are created using recombinant DNA technology which involves manipulating and isolating DNA segments.
Peptides in pharmaceuticals are also often referred to as therapeutic peptides. They are most commonly used for the treatment of diseases. Some examples of lab-produced peptides are human insulin and growth hormone, both of which can be life-saving for people living with certain conditions.
Getting More Specific: What Are the Benefits of Peptides in Pharmaceuticals?
The development of peptides in pharmaceuticals has made a lot of progress in the last decade thanks to new production, modification, and DNA technologies. Some of the most notable benefits of developing and utilizing pharmaceutical peptides include:
- Low production costs and sale prices
- Oral administration allows for easy delivery to patients
- Good membrane penetration ability
There aren’t many cons when it comes to utilizing pharmaceutical peptides in the medial field, but one that should be noted is they tend to be short lived in the body as a result of rapid degradation and clearance.
Our Capabilities in Pharmaceutical Peptide Production
Oakwood Labs has decades of experience in researching and manufacturing a wide range of pharmaceuticals, including pharmaceutical peptides. Our international reputation for pharmaceutical development is all thanks to our highly skilled, innovative, and knowledgeable staff.
When it comes to utilizing peptides in pharmaceuticals, our team is highly equipped to perform research and lead in the development of individually formulated products for your specific needs.
Oakwood Labs Is an Industry Leader
In addition to manufacturing pharmaceutical peptides, which, as we’ve highlighted, are crucial in the management of chronic diseases, Oakwood Labs provides many other products and services that cater to the medical field. Some of those services include:
When you work with Oakwood Labs, you can expect high-quality results every time. We maintain rigorous quality assurance and validation practices to provide the absolute best solutions for our customers. The partnerships we’ve long maintained are attributed to our skilled team and history of compliance.
Reach Out to Us for Pharmaceutical Peptides and More
Oakwood Labs is your source for R&D formulation development through GMP commercial manufacturing. If you’re interested in learning more about our capabilities when it comes to peptides in pharmaceuticals, contact our team today.
The utilization of biodegradable polymers has come a long way during the past few decades. As science and engineering continue to evolve, so do the advancements with utilizing biodegradable polymers in drug delivery. Let’s examine some key aspects.
Starting with the Basics: What Are Biodegradable Polymers?
A biodegradable polymer is engineered to deteriorate over time. These specific polymers have a high molecular weight that depreciates to a lower molecular weight after its intended function. Essentially, once these polymers have served their purpose with appropriate response from the host, they are broken down and eliminated from the body.
Biodegradable polymers are produced both naturally and synthetically. The two most common natural biodegradable polymers include proteins and polysaccharides. Synthetic biodegradable polymers consist of amides, ethers, urethanes, and other esters.
PLA and PLGA are two common biocompatible and biodegradable polymers. These FDA-approved polymers have been used in medical devices, such as microspheres. One of the major benefits of working with biodegradable polymers, such as PLA and PLGA, is that they can be used to encapsulate just about any type of drug, including:
- Proteins
- Peptides
- Polypeptides
- Small molecules
In addition to using biodegradable polymers in controlled drug delivery, they can also be used for the following applications:
- Gene delivery
- Wound dressings
- Tissue regeneration and engineering
- Cryopreservation
- Enzyme immobilization
- Nanotechnology
- Orthopedic fixation devices
- Prosthetics
- Biomedical devices and implants
- Cosmetics
- Augmentation
- Stitches
Why Are Biodegradable Polymers Effective?
Whether biodegradable polymers are natural or synthetic, they have unique biocompatible properties that allow them to decompose inside the body by natural, nontoxic byproducts such as carbon dioxide or water. One benefit to synthetic biodegradable polymers is that they can be produced with a wide range of mechanical, physical, and chemical properties that can be modified. Because these specific polymers can be synthesized by reproducing the same polymer, they have endless availability.
Naturally occurring biodegradable polymers are just as effective as synthetic in that they offer temporary support and aid in controlled release factors throughout the body. Some other major advantages of using biodegradable polymers in drug delivery include:
- Ability to adjust the degradation rates and mechanical properties to form into various configurations
- Decreased need for successive surgical removal, which overall saves money and time
- Sterilizable properties that can be easily processed by the host
- Modifiable surfaces
Biodegradable Polymers at Oakwood Labs
Oakwood Labs is a leader in the pharmaceutical industry providing manufacturing services and contract development. Our state-of-the-art technology allows us to be experts with biodegradable polymers in drug delivery.
At Oakwood labs, we utilize biodegradable polymers such as PLGA, PLA, and other polymers that demonstrate low toxicity. These biodegradable polymers are used in our long acting injectables, which are based upon our technology platform Chroniject™. Our patented microsphere technology is a polymer-based injectable microsphere system for drug delivery.
Other advantages of our technology used for biodegradable polymers in drug delivery include:
- Sourced from multiple suppliers
- Quick and effective development of formulations using small scale batches
- Easily scalable formulations
- Controlled process parameters
- Proven lot-to-lot reproducibility
- Molecule compatibility (peptides, proteins, and small molecules)
- Adjustable release durations ranging from one week to one year
- Exceptional stability
- Immediate reconstitution with WFI with no special diluent needed
- Versatile use in multiple therapeutic applications (neurology, depression, CNS, ophthalmology)
Contact Us Regarding Our Biodegradable Polymers in Drug Delivery
At Oakwood Labs, we have over 20 years of experience with drug encapsulation, which has propelled us to being a leader in sustained release drug delivery. When it comes to using biodegradable polymers in drug delivery, our advanced technology provides the ability to customize release duration ranging from weeks to years.
If you’re interested in learning more about biodegradable polymers, reach out to our team today.
When pharmaceutical companies set out to create new drugs or modify existing ones for innovative applications, the process is necessarily complex, intricate, and thorough. Since any medication goes beyond its status as a product and affects the health and wellbeing of patients, ensuring that these drugs are held to rigorous quality standards and are produced within facilities adhering to the same is essential.
At Oakwood Labs, our dedicated team supplies support for all phases of long-acting injectable development, facilitating a systematic technology transfer process that ensures our clients’ products are delivered safely and securely.
About Technology Transfer for Pharmaceuticals
Technology transfer refers to the process of new inventions and innovations being transformed into products ready for commercialization. While much of the work for these inventions and innovations can occur within lab settings, the results are often intellectual property owned by and created on behalf of corporations or start-ups.
Because of this, the technology transfer process for pharmaceuticals and other focuses seeks to protect the intellectual property of these institutions so that their products can be properly commercialized and monetized, while benefitting medical facilities and patients at large.
Other aspects that can be components of technology transfer for pharmaceuticals include the following:
- Working alongside legal departments securing patents and other rights
- Determining commercial viability of innovations
- Advertising technologies to varied parties
- Teaching principles of and strategizing for commercialization
- Obtaining funding for research
- Planning licensure agreements
- Strategizing business plans for product implementation
As you can see, technology transfer encompasses a wide array of aspects and Oakwood Labs is proud to play a vital role in ensuring that products are crafted to institutions’ specifications and expectations. This helps to guarantee that innovative medicines can advance to further stages and be brought to market, bringing varied advantages to both companies and patients.
Beyond the Technology Transfer Process: Everything That Oakwood Labs Can Provide
When you partner with Oakwood Labs, you gain access to a team with over 20 years of drug encapsulation experience who has provided services for global pharmaceutical firms of diverse sizes. Our expertise in sustained-release drug delivery allows us to provide the reliable, injectable microsphere-based formulations that our clients require.
We are able to supply a host of services and benefits for our clients, such as:
- Chroniject™, our patented microsphere-based technology
- R&D formulation development
- Efficient and stringent process controls
- Tailored release profile from weeks to one year
- GMP manufacturing under rigorous in-house quality systems
- Contract manufacturing services
- An FDA-approved, aseptic GMP facility
- Demonstrated lot-to-lot reproducibility with proven scale-up success
- A strong focus on communication and project management
- A track record of success for taking projects from proof of concept to clinical trials
If you’ve been searching for a trustworthy lab to work on your project and facilitate technology transfer for pharmaceuticals, choose Oakwood Labs. We have the facilities, expertise, and team needed to deliver.
Reach Out to Oakwood Labs Today
To ask our team questions about our processes, or to get started on a project, feel free to contact us. We’ll discuss your goals with you and help determine the best approach to realizing them.