Archives by Year: 2022

Key Takeaways About the Stages of Drug Development
- The pharmaceutical development process is a structured, multi-step procedure involving discovery, preclinical testing, clinical research, FDA review, and post-market monitoring, all aimed at ensuring safety and effectiveness.
- Each pharmaceutical phase serves a distinct purpose, from identifying promising compounds and testing them in controlled environments to confirming results in human trials and evaluating overall effectiveness.
- Regulatory standards play a critical role in the stages of drug development, with the FDA overseeing not only the approval process through requirements like new drug applications but also ongoing monitoring once drugs are on the market.
- Scientific rigor and safety remain central throughout, as researchers evaluate dosage, side effects, interactions, and long-term impact to bring reliable treatments to patients.
Designed to work as a long, persistent, and rigorous process, drug development is crucial to progressing the field of medicine. Drug researchers and developers are tasked with expanding on previous information and findings in order to combat the rise of new, as well as prevalent, diseases.
Because of this, our team at Oakwood Labs wants to highlight the five drug development stages to further encapsulate the rigor of the process of providing sustained-released injectable pharmaceuticals for the community.
A Brief Introduction to Pharmaceutical Phases
As addressed by the FDA, the stages of drug development follow a similar trend for each case. Because of this, we have outlined each drug development stage below, as we feel it is important for our clientele to have an inside look at the processes that we adhere to.
Discovery and Development
Known as the first stage of drug development, the discovery and development stage starts with an initial idea that is fabricated through consistent research and trends. During this stage, scientists discover new insights into the disease process, which allows them to develop a design for a product to either stop or reverse the effects of the disease under consideration. From there, several tests of the molecular compounds are tested to find the possible beneficial effects, or even test existing treatments that might possibly have unanticipated effects.
Once researchers find results of a promising compound through the discovery process, they conduct further experiments to gather information on the following hypotheses:
- How the promising compounds absorb, distribute, metabolize, and dispose of waste
- The mechanisms of action, and how they can be beneficial to further drug development
- The best dosage of the future drug
- The best way to give or take the drug, such as by mouth or as an injectable
- The side effects of the drugs, also known as toxicity
- How it affects individuals of different races, genders, and ethnicities differently
- How it correlates and interacts with other drugs and treatments
- Its overall effectiveness compared to similar treatments
After conducting the series of tests to gauge additional information, researchers are then ready to conduct the preclinical research stage of drug development.
Preclinical Research
As a precautionary measure, researchers must further test their drug for toxicity before distributing the finalized product for public use. Presented in the form of either in vitro or in vivo research, this stage of drug development must be conducted in favor of good laboratory practices (GLP), which can be found in 21 CFR Part 58.1: Good Laboratory Practice for Nonclinical Laboratory Studies. These regulations essentially set the minimum basic requirements for features, such as study conduct, personnel, facilities, equipment, written protocols, and operating procedures, among others.
Though preclinical studies are not usually large, they must provide very stringent and detailed information regarding dosing and overall toxicity levels. Once completed, researchers will assess their findings and decide whether the drug should proceed to the clinical research stage of drug development.
Clinical Research
During this drug development stage, the drug that was initially tested in the preclinical trial will now be tested on people with the hopes of finding how it will interact with the human body. But before the clinical trial can even start, developers will need to design the clinical study for the trial.
When designing a clinical trial, the drug development team follows a specific study plan or protocol that is initially developed by the researcher or manufacturer. Then, the researchers will review the information from the previous two stages to develop research questions, objectives, and decide the following:
- A selection criterion of who qualifies to participate
- The number of people allotted for the study
- The duration of the study
- If there should be the presence of a control group to limit research bias
- How and at what dosage the drugs will be administered
- What assessments will be conducted and what data will be collected
- How the collected data will be reviewed and analyzed for future drug development stages
Unlike the preclinical trials, the clinical trials range from early, small-scale studies to late-stage, large-scale studies.
FDA Review
Considered the final drug development stage, the FDA review team will thoroughly examine the evidence from the drug’s early tests, preclinical and clinical research, and decide to either approve or not to approve the product.
During this stage, a New Drug Application (NDA) must be provided to outline the entirety of the drug development stages that lead to the anticipated approval. The NDA must include the following when presented:
- Proposed labeling
- Safety updates
- Drug abuse information
- Patent information
- Any data of studies that were not conducted within the United States
- Institutional review board compliance information
- Clear and concise directions for use
Finally, the drug is sent for approval to the FDA, where the committee decides whether the drug is safe and effective for public use. The process of approval can take anywhere from 6 to 10 months, as it goes through several inspectors, project managers, and pharmacologists.
From there, the drug is passed to the labeling and marketing stage, where the remaining issues will need to be resolved, and labeling will be provided accurately and objectively for public discretion.
FDA Post-Market Safety Monitoring
Though there are rigorous and persistent steps during each stage of drug development up to its approval and distribution, limitations are still evident and existent. Because of this, the FDA closely monitors drugs for months and even years so they can review the reports on the prescription and decide whether or not to add cautions to the dosage or usage information, which can still be seen as part of the pharmaceutical development process.
If there were significant changes that needed to be made to a product after the drug development stages were complete, developers would have to file a supplemental application in order to make those changes.
Why Choose Us for Your Pharmaceutical Needs
At Oakwood Labs, our drug development team is honored to provide sustained-released injectables to our extensive clientele. On top of that, some of the benefits of working with our pharmaceutical company through each stage of drug development include the following:
- Pre-formulation development
- Feasibility studies
- Scaling formulation
- Toxicology batch manufacturing
- ICH compliant stability tests
- Small-scale to large-scale clinical trial manufacturing
- Commercial and aseptic GMP manufacturing
Contact Oakwood Labs to Learn More About the Stages of Drug Development
If you have any specific questions regarding the drug development stages that Oakwood Labs conducts for sustained-release pharmaceuticals, please contact our team today. We look forward to assisting you in any way we can.
In order for injectables to be able to successfully provide sustained releases, they necessitate drug-encapsulating devices. For over 50 years, encapsulation has been one of the many techniques used in the pharmaceutical industry. In this process, medication is released from a microsphere by the drug detaching from the polymer or through the degradation of the polymer matrix.
Keep reading to learn more about the role of polymer microspheres in the process of drug delivery and how our technology can be an asset to your long-acting injectable project.
The Use of Polymer Microspheres in the Pharmaceutical Industry
Polymer microspheres are often used in drug delivery because they have a high surface area and low particle size, which increases their absorption rate and bioavailability. Since these microspheres are small in size, they are also efficient when moving throughout the body. They are additionally capable of sustained release, making them ideal for use in long-acting injectables for both passive and active targeting.
Capabilities of Microspheres
Microspheres can be organic or inorganic and are designed to encapsulate bioactive molecules and release them in a controlled way. They are used for desired release profiles and can be used to target specific delivery sites throughout the body, including organs.
Additionally, they are considered rigid which means that they can be packed together, found alone, or even combined with other biomaterials to create porous 3D-structured scaffolds. In these scaffolds, they can either serve as a component of a larger scaffold or be the building blocks of one.
Factoring in the Polymer
Polymers can take the role of bioactive or biodegradable depending on the agents they are combined with. When incorporated with therapeutics, polymers become bioactive and provide their own therapeutic benefit. However, they can also be biodegradable which improves release kinetics and helps prevent carrier accumulation.
The benefits of using polymers for encapsulation include the following:
- Ability to encapsulate a variety of drugs
- They have high biocompatibility
- They are bioavailable
Incorporating ChronijectTM Technology into Your Project
Since polymer microspheres are suited for sustained-release technology, they are the perfect candidate for long-acting injectables. Our technology for microspheres is a patented, polymer-based injectable for drug delivery called ChronijectTM. This technology is compatible with small molecules, peptides, and proteins. It is also capable of enabling sustained release over durations of two weeks to one year.
Our ChronijectTM process for microsphere manufacturing initially uses small batch sizes to enable the production of numerous trial batches. This allows us to obtain a formation with the desired release profile and other indicated product characteristics. Additionally, this process can easily be scaled up to commercial production levels with lot-to-lot reproducibility. Since we can produce clinical trial material at the forecast commercial scale, scaling up is feasible at the commercial scale and is conducted in our GMP facility.
Advantages of Using ChronijectTM
There are diverse advantages of using polymer microspheres and our ChronijectTM technology for drug delivery, which include the following:
- Rapid development of formulations using small-scale batches
- Easily scaled formations
- Process parameters that are monitored and controlled
- Proven lot-to-lot reproducibility
- Molecule compatibility (small molecules, peptides, proteins)
- Flexible release durations, ranging from one week to one year
- Excellent stability
- Instant reconstitution with WFI – no special diluent required
- Applications in various therapeutic indications (CNS, neurology, ophthalmology)
About Oakwood Labs
Oakwood Labs is a specialty pharmaceutical company located near Cleveland, Ohio. We are focused on developing and manufacturing sustained-release injectable pharmaceuticals, incorporating our microsphere-based technology. We operate in a fully compliant, aseptic facility that is specifically used for manufacturing pharmaceutical products like injectables.
Our goal is to provide enhanced therapeutic benefits to patients while achieving excellent returns for our pharmaceutical partners. With over 20 years of experience, we have proven ourselves as an effective partner of global pharmaceutical firms. From proof of concept and feasibility studies to clinical trials and FDA-approved supplies, the team at Oakwood Labs is ready to help incorporate polymer microspheres into the development of long-acting injectables.
Contact Our Team with Any Questions
If you are interested in learning more about our capabilities in incorporating polymer microspheres into long-acting injectables, please reach out to our team today.
What Are Peptides?
Peptides are strings of amino acids, also known as the “building blocks” of proteins. The human body naturally produces more than 7,000 known peptide types. Along with the peptides that are naturally produced by the human body, they can also be produced in medical laboratories. Pharmaceutical peptides are those that are created using recombinant DNA technology which involves manipulating and isolating DNA segments.
Peptides in pharmaceuticals are also often referred to as therapeutic peptides. They are most commonly used for the treatment of diseases. Some examples of lab-produced peptides are human insulin and growth hormone, both of which can be life-saving for people living with certain conditions.
Getting More Specific: What Are the Benefits of Peptides in Pharmaceuticals?
The development of peptides in pharmaceuticals has made a lot of progress in the last decade thanks to new production, modification, and DNA technologies. Some of the most notable benefits of developing and utilizing pharmaceutical peptides include:
- Low production costs and sale prices
- Oral administration allows for easy delivery to patients
- Good membrane penetration ability
There aren’t many cons when it comes to utilizing pharmaceutical peptides in the medial field, but one that should be noted is they tend to be short lived in the body as a result of rapid degradation and clearance.
Our Capabilities in Pharmaceutical Peptide Production
Oakwood Labs has decades of experience in researching and manufacturing a wide range of pharmaceuticals, including pharmaceutical peptides. Our international reputation for pharmaceutical development is all thanks to our highly skilled, innovative, and knowledgeable staff.
When it comes to utilizing peptides in pharmaceuticals, our team is highly equipped to perform research and lead in the development of individually formulated products for your specific needs.
Oakwood Labs Is an Industry Leader
In addition to manufacturing pharmaceutical peptides, which, as we’ve highlighted, are crucial in the management of chronic diseases, Oakwood Labs provides many other products and services that cater to the medical field. Some of those services include:
When you work with Oakwood Labs, you can expect high-quality results every time. We maintain rigorous quality assurance and validation practices to provide the absolute best solutions for our customers. The partnerships we’ve long maintained are attributed to our skilled team and history of compliance.
Reach Out to Us for Pharmaceutical Peptides and More
Oakwood Labs is your source for R&D formulation development through GMP commercial manufacturing. If you’re interested in learning more about our capabilities when it comes to peptides in pharmaceuticals, contact our team today.
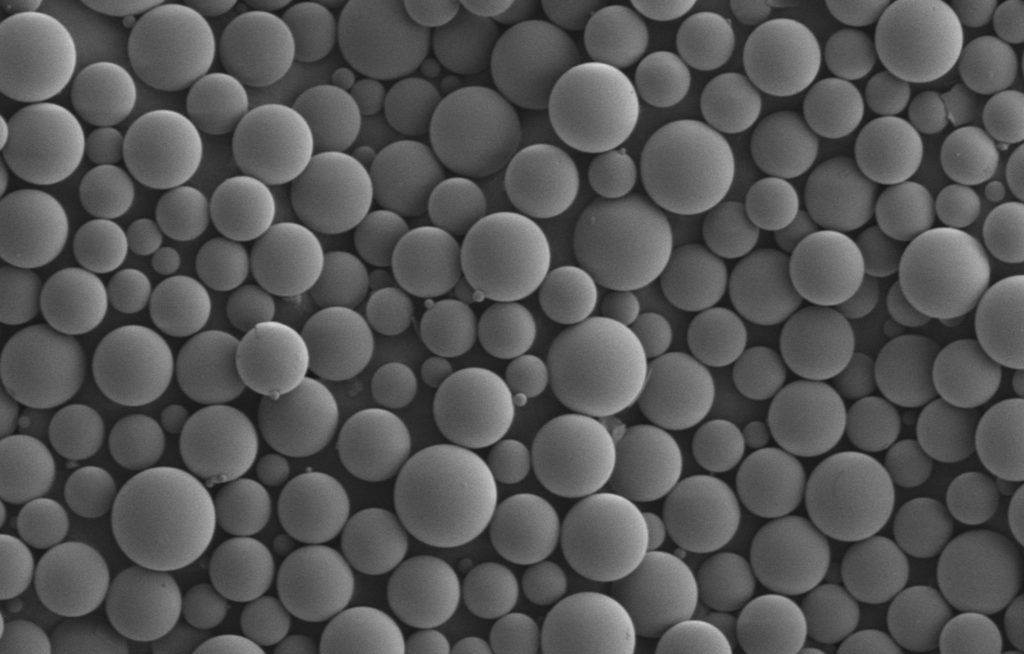
The utilization of biodegradable polymers has come a long way during the past few decades. As science and engineering continue to evolve, so do the advancements with utilizing biodegradable polymers in drug delivery. Let’s examine some key aspects.
Starting with the Basics: What Are Biodegradable Polymers?
A biodegradable polymer is engineered to deteriorate over time. These specific polymers have a high molecular weight that depreciates to a lower molecular weight after its intended function. Essentially, once these polymers have served their purpose with appropriate response from the host, they are broken down and eliminated from the body.
Biodegradable polymers are produced both naturally and synthetically. The two most common natural biodegradable polymers include proteins and polysaccharides. Synthetic biodegradable polymers consist of amides, ethers, urethanes, and other esters.
PLA and PLGA are two common biocompatible and biodegradable polymers. These FDA-approved polymers have been used in medical devices, such as microspheres. One of the major benefits of working with biodegradable polymers, such as PLA and PLGA, is that they can be used to encapsulate just about any type of drug, including:
- Proteins
- Peptides
- Polypeptides
- Small molecules
In addition to using biodegradable polymers in controlled drug delivery, they can also be used for the following applications:
- Gene delivery
- Wound dressings
- Tissue regeneration and engineering
- Cryopreservation
- Enzyme immobilization
- Nanotechnology
- Orthopedic fixation devices
- Prosthetics
- Biomedical devices and implants
- Cosmetics
- Augmentation
- Stitches
Why Are Biodegradable Polymers Effective?
Whether biodegradable polymers are natural or synthetic, they have unique biocompatible properties that allow them to decompose inside the body by natural, nontoxic byproducts such as carbon dioxide or water. One benefit to synthetic biodegradable polymers is that they can be produced with a wide range of mechanical, physical, and chemical properties that can be modified. Because these specific polymers can be synthesized by reproducing the same polymer, they have endless availability.
Naturally occurring biodegradable polymers are just as effective as synthetic in that they offer temporary support and aid in controlled release factors throughout the body. Some other major advantages of using biodegradable polymers in drug delivery include:
- Ability to adjust the degradation rates and mechanical properties to form into various configurations
- Decreased need for successive surgical removal, which overall saves money and time
- Sterilizable properties that can be easily processed by the host
- Modifiable surfaces
Biodegradable Polymers at Oakwood Labs
Oakwood Labs is a leader in the pharmaceutical industry providing manufacturing services and contract development. Our state-of-the-art technology allows us to be experts with biodegradable polymers in drug delivery.
At Oakwood labs, we utilize biodegradable polymers such as PLGA, PLA, and other polymers that demonstrate low toxicity. These biodegradable polymers are used in our long acting injectables, which are based upon our technology platform Chroniject™. Our patented microsphere technology is a polymer-based injectable microsphere system for drug delivery.
Other advantages of our technology used for biodegradable polymers in drug delivery include:
- Sourced from multiple suppliers
- Quick and effective development of formulations using small scale batches
- Easily scalable formulations
- Controlled process parameters
- Proven lot-to-lot reproducibility
- Molecule compatibility (peptides, proteins, and small molecules)
- Adjustable release durations ranging from one week to one year
- Exceptional stability
- Immediate reconstitution with WFI with no special diluent needed
- Versatile use in multiple therapeutic applications (neurology, depression, CNS, ophthalmology)
Contact Us Regarding Our Biodegradable Polymers in Drug Delivery
At Oakwood Labs, we have over 20 years of experience with drug encapsulation, which has propelled us to being a leader in sustained release drug delivery. When it comes to using biodegradable polymers in drug delivery, our advanced technology provides the ability to customize release duration ranging from weeks to years.
If you’re interested in learning more about biodegradable polymers, reach out to our team today.
When pharmaceutical companies set out to create new drugs or modify existing ones for innovative applications, the process is necessarily complex, intricate, and thorough. Since any medication goes beyond its status as a product and affects the health and wellbeing of patients, ensuring that these drugs are held to rigorous quality standards and are produced within facilities adhering to the same is essential.
At Oakwood Labs, our dedicated team supplies support for all phases of long-acting injectable development, facilitating a systematic technology transfer process that ensures our clients’ products are delivered safely and securely.
About Technology Transfer for Pharmaceuticals
Technology transfer refers to the process of new inventions and innovations being transformed into products ready for commercialization. While much of the work for these inventions and innovations can occur within lab settings, the results are often intellectual property owned by and created on behalf of corporations or start-ups.
Because of this, the technology transfer process for pharmaceuticals and other focuses seeks to protect the intellectual property of these institutions so that their products can be properly commercialized and monetized, while benefitting medical facilities and patients at large.
Other aspects that can be components of technology transfer for pharmaceuticals include the following:
- Working alongside legal departments securing patents and other rights
- Determining commercial viability of innovations
- Advertising technologies to varied parties
- Teaching principles of and strategizing for commercialization
- Obtaining funding for research
- Planning licensure agreements
- Strategizing business plans for product implementation
As you can see, technology transfer encompasses a wide array of aspects and Oakwood Labs is proud to play a vital role in ensuring that products are crafted to institutions’ specifications and expectations. This helps to guarantee that innovative medicines can advance to further stages and be brought to market, bringing varied advantages to both companies and patients.
Beyond the Technology Transfer Process: Everything That Oakwood Labs Can Provide
When you partner with Oakwood Labs, you gain access to a team with over 20 years of drug encapsulation experience who has provided services for global pharmaceutical firms of diverse sizes. Our expertise in sustained-release drug delivery allows us to provide the reliable, injectable microsphere-based formulations that our clients require.
We are able to supply a host of services and benefits for our clients, such as:
- Chroniject™, our patented microsphere-based technology
- R&D formulation development
- Efficient and stringent process controls
- Tailored release profile from weeks to one year
- GMP manufacturing under rigorous in-house quality systems
- Contract manufacturing services
- An FDA-approved, aseptic GMP facility
- Demonstrated lot-to-lot reproducibility with proven scale-up success
- A strong focus on communication and project management
- A track record of success for taking projects from proof of concept to clinical trials
If you’ve been searching for a trustworthy lab to work on your project and facilitate technology transfer for pharmaceuticals, choose Oakwood Labs. We have the facilities, expertise, and team needed to deliver.
Reach Out to Oakwood Labs Today
To ask our team questions about our processes, or to get started on a project, feel free to contact us. We’ll discuss your goals with you and help determine the best approach to realizing them.
3 Things to Know About the Types of Long-Acting Injectables
- Common drugs used as long-acting injectable antipsychotics include fluphenazine, haloperidol decanoate, and risperidone.
- Long-acting injectables gradually release medication into the bloodstream and can last between two to twelve weeks per dose. They allow providers to monitor treatment closely through routine appointments, supporting social interaction and building a medical routine.
- Use of long-acting injectables reduces side effects compared to daily pills, resulting in steadier plasma drug levels and better cognitive and functional outcomes.
In the world of medicine, there are several methods by which clinical professionals administer drugs to their patients for the purposes of treatment, recovery, illness prevention, and/or relief. The most widely known methods of administering drugs are orally, topically, and via injections.
Choosing the most effective method is based on many different factors but include the patient and their medical history, desired outcome, type of drug, and more. Long-acting injectables are one method of drug administration that is particularly favorable among clinical professionals due to the convenience and effectiveness. Below, we’ll review the benefits of different types of long-acting injectables and how they are developed and manufactured.
What Is a Long-Acting Injectable?
A long-acting injectable is an injection method which allows for the gradual release of medication into the bloodstream. There are different types of long-acting injectables, and their effects can last anywhere from two to twelve weeks with just one dose depending on the medication.
Different types of long-acting injectables are commonly used as alternative treatments for those individuals who have trouble taking daily or regular medicines in the form of liquids, tablets, or capsules.
Different Types of Long-Acting Injectables
Since their introduction into clinical practices in the early 1960s, long-acting injectable antipsychotic drugs have been widely used as maintenance therapy and a reliable treatment option for those with psychotic disorders, such as schizophrenia.
These different types of long-acting injectables vary in terms of how often they need to be administered as well as how long their medical benefits will last. A few of the most commonly administered long-acting injectable antipsychotic drugs used today include:
- Fluphenazine
- Haloperidol decanoate
- Risperidone
Answering the Question: Why Are Different Types of Long-Acting Injectables Important?
Long-acting injectable drugs allow for the slow release of medicine into the blood and their effects can last anywhere from two to twelve weeks, making them a practical option for those who have trouble taking medicine on a regular or daily basis. Other benefits of these different types of long-acting injectables include:
- No need to remember to take medications every day
- Routine interactions between patient and medical staff for injection visits
- Overall reduced relapse frequency and rehospitalization rates
- Reduces the risk of accidental or purposeful overdose
- Treating patients with more stable plasma concentrations than oral medications
Considerations When Utilizing Long-Acting Injectables
As with any clinical solution or medication, there are potential side effects to these different types of long-acting injectables that are dependent upon various factors. Some considerations to these drug alternatives may include:
- Prolonged period to achieving steady-state levels
- Slow dose titration
- Less flexibility of dose adjustment
- Delayed desertion of distressing and/or severe side effects
- Pain at the injection site can occur
- Leakage into the subcutaneous tissue and/or the skin can cause irritation
- Burden of frequent travel to outpatient clinics or home visits by community nurses for their administration
- Perception of stigma
Many Different Types of Long-Acting Injectables Can Be Manufactured at Oakwood Labs
Oakwood Labs is a leading developer of sustained-release pharmaceuticals with over 20 years of drug encapsulation experience. We offer support throughout the development of different types of long-acting injectable, ultimately achieving a product that provides greater remedial benefits to patients around the world.
Our facilities can provide the following to help achieve your project goals, including those involving various types of long-acting injectable:
- Pre-formulation development
- Feasibility studies
- Scaling formulation
- Toxicology batch manufacturing
- ICH compliant stability tests
- Phase I, II, and III clinical trial manufacturing
- GMP manufacturing (commercial and aseptic)
Contact Us Today to Learn More!
Long-acting injectables are a proven method to preventing the intense symptoms associated with mental illnesses and psychotic disorders. Using this clinical method has the ability to put patients on the path to a more stable and prosperous life.
The team at Oakwood Labs is ready to assist in the development and manufacturing of different types of long-acting injectable drugs. Reach out today to get started on your next project with us.
We look forward to working with you!

The first step is conducted through two different studies. At Oakwood Labs, we first select a target formulation to receive tests during the feasibility study and then again with the in vivo animal study. After that is complete, it’s then time for the scale-up process to commence.
Understanding what pharmaceutical scale-up is and the importance of the process in the pharmaceutical industry can help businesses increase the number of products that are growing in demand.
Defining the Scale-Up Process
The scale-up process is designed to increase the amount of an existing product, taking production from a smaller scale to a larger scale during a short duration of time.
Our pharmaceutical scale-up process involves increasing the development of the formulation while still making the same product attributes as we normally would. Through the scale-up process in pharmaceuticals, we offer our clients quality assurance and validations to any concerns they may have.
Important Factors to Consider for the Scale-Up Process in the Pharmaceutical Industry
When tested drugs are ready for the pharmaceutical scale-up process, there are important factors to consider. To make sure your product is in an ideal position for market success, there is a need for focused and careful planning. Also, when drugs are first going through the pharmaceutical scale-up process they are continuously being optimized. Within our continuous flow process, the equipment we use to manufacture prototype formulations is the same that is used to manufacture full-scale lots, facilitating an easy transition and ensuring the process goes smoothly.
Familiarizing Yourself with the Different Scale Stages
There are different stages of the pharmaceutical scale-up process that drugs undergo. When a drug is in the first stages of receiving tests, it is at the laboratory-scale level. Pilot scale follows and is designed for the purpose of expanding clinical trials. Production scale for commercial use is the final scale in the cycle and is implemented when a new drug is approved. At Oakwood Labs, we can guide your product through the needed scales, providing consistency and reproducibility that lead to the best results.
Contact Us Regarding Our Scale-Up Process in Pharmaceuticals
If you’re in need of scale-up for your pharmaceutical products, be sure to reach out. We are an industry leader in sustained-release injectable pharmaceutical development and manufacturing, and we are committed to supplying products that provide enhanced therapeutic benefits to patients.
Contact us to learn more about the pharmaceutical scale-up process or our other manufacturing capabilities.
When caring for patients, it is critical to use medical treatments that are not only effective but also seamlessly incorporate into their lifestyles. With the risks associated with pills and the need for a regular routine, medical personnel often will turn to long-acting injectable antipsychotics for patient treatment due to the approach’s benefits.
Long-acting injectable antipsychotic drugs are commonly used for patients who experience unpleasant adverse side effects of antipsychotic drugs and are hesitant to believe they have a mental illness. While this form of medicine does not require the daily administration that other antipsychotics do, they are able produce relief to patients in need.
Administering Long-Acting Injectable Antipsychotics
Long-acting injectable antipsychotics can be utilized with diverse medications, making them ideal for patients suffering from an assortment of issues. Along with this, long-acting injectable antipsychotics are often administered in two-, three- or four-week intervals, but treatments can vary based on the patient’s needs. Time between injections also differs based upon the administered drug and its ability to stay within the system.
Candidates for this treatment often include patients with a history of non-adherence, severe symptoms, comorbid substance abuse, and cognitive impairment. Additionally, this method is extremely beneficial to patients who experience ambivalence or negative attitudes toward medications.
Why Long-Acting Injectable Antipsychotics Are Ideal for Patients
There are many advantages to using long-acting injectable antipsychotics on eligible individuals, as they can improve the lives and routines of patients in the following ways:
- Reduced accountability – In order for medications to work best, it is important for patients to take them every day according to instructions. Since long-acting injectable antipsychotics stay in the system for extended periods of time, they reduce the need for a daily reminder to take the drug.
- Regularity – By using long-acting injectable antipsychotics, the need for medical appointments increases. This creates regular interactions between the patient and medical staff, creating a more comfortable environment, providing more social interactions, and solidifying a routine.
- Stability – The long-acting injectable method treats patients with more stable plasma concentrations than oral medications. It also lessens the frequency of having peak plasma levels, which can result in fewer side effects for the patient. Lastly, it reduces relapse frequency and rehospitalization rates, making it a great alternative for those who are prone to hospital visits already.
- Safety – Since long-acting injectable antipsychotic drugs are administered by medical personnel, they are safer for patients. The approach reduces the risk of accidental or deliberate overdose through the use of regulated injections that work gradually over time.
Oakwood Labs Is Ready to Help You Produce Long-Acting Injectable Antipsychotics and More
Oakwood Labs is a leading manufacturer of sustained-release pharmaceuticals with over 20 years of drug encapsulation experience. We offer support throughout the phases of long-acting injectable development, ultimately achieving a product that provides enhanced therapeutic benefits to patients everywhere.
In our facilities, we can provide the following to help achieve your project goals, including those involving long-acting injectable antipsychotics:
- Pre-formulation development
- Feasibility studies
- Scaling formulation
- Toxicology batch manufacturing
- ICH compliant stability tests
- Phase I, II, and III clinical trial manufacturing
- GMP manufacturing (commercial and aseptic)
Contact Us Today to Learn More!
Long-acting injectable antipsychotics are a way to steadily prevent the intense symptoms associated with mental illnesses. Using this approach has the ability to put patients on the path to a stable and successful life.
The team at Oakwood Labs is ready to help with the development of your long-acting injectable antipsychotic drugs. Please reach out today and get started on your project with us!
3 Key Takeaways
- Phase 3 trials generate regulatory-grade data essential for drug approval, involving rigorous study protocols and safety monitoring by independent boards such as Data and Safety Monitoring Boards (DSMBs).
- Designing Phase 3 trials involves scaling up drug production to commercial levels, validating processes through stability studies, and ensuring data robustness to avoid commercialization delays if successful.
- Post-trial, researchers analyze whether the drug meets pre-specified endpoints related to safety, efficacy, and patient quality of life, guiding regulatory approval decisions.
Clinical trials are defined as research studies that involve people.
A Phase 3 clinical trial is the stage at which a new drug is tested for efficacy and adverse reactions on a group of volunteers ranging in size from 300 to 3,000.
The Phases of a Clinical Trial
- Phase 1 – Testing the safety and dosage of a new drug, the first phase of a clinical trial is conducted on a pool of 20 to 100 volunteers over the course of several months. Approximately 70% of drugs move on to Phase 2.
- Phase 2 – Conducted over the course of a few months up to a few years, Phase 2 of a clinical trial is meant to test the efficacy and side effects of a new drug on a group ranging from 100 to several hundred people.
- Phase 3 – Phase 3 clinical trials are conducted on 300 to 3,000 volunteers over the course of one to four years. This stage continues to test for the efficacy of a new drug while monitoring for adverse reactions.
- Phase 4 – In the final phase, several thousand volunteers undergo clinical trials to further test for the safety and efficacy of the medicine.
Phase 3 Clinical Trial – Efficacy and Monitoring Adverse Reactions
With a length of one to four years, a Phase 3 clinical trial is more likely to show researchers long-term or rare side effects to a medication. This is the phase in which most of the safety data about the new drug is gathered, as it’s possible that less-common side effects have failed to be detected in earlier, shorter phases of the clinical trial up to this point.
Phase 3 clinical trials are designed by researchers to show whether or not a product is a beneficial treatment to a specific population of people. Only 25-30% of drugs move on to Phase 4.
Designing a Phase 3 Clinical Trial
At Oakwood Labs, we have extensive experience in producing global clinical trial material for Phase 1, 2, and 3 clinical trials. Through our Microbiology and Chemistry groups, we offer full characterization capabilities – proper documentation of batch release and accelerated and long-term stability studies.
As our team makes progress in development, the process will be validated. Multiple lots of API and polymer are used in the Phase 3 clinical trial process to demonstrate the product. We recommend conducting Phase 3 clinical trials on the same scale as intended for commercial scale to avoid delays in commercialization if clinical trials are successful.
Key Elements of a Phase 3 Clinical Trial
Regulatory agencies rely on the data derived from Phase 3 clinical trials, so it’s essential that these trials meet rigorous standards. Some key elements that make up a Phase 3 clinical trial include:
- Large sample size – You can expect a Phase 3 clinical trial to involve a large group of participants, such as hundreds to thousands of people, in order to provide statistically meaningful results and show differences between treatments.
- Multi-site execution – These trials are conducted across various locations such as in hospitals or clinics, but also could be conducted internationally. This ensures that findings can be applied to diverse populations.
- Blinding protocols – Ideally, these trials should be double-blind, meaning that neither the clinician nor patient knows which treatment is being given. This ensures there is no bias involved in procedures and that the process is fully randomized.
- Safety monitoring – All Phase 3 trials require monitoring by a data and safety monitoring board (DSMB) since these trials are large and conducted at various sites.
How Long Does a Phase 3 Clinical Trial Last?
There is no requirement when it comes to a timeline for Phase 3 clinical trials, but they are often longer and require a follow-up. You can expect the trial to last 1–4 years. The extended length is due to the comprehensive tests conducted during this phase to evaluate the drug’s long-term effects.
Ensure Quality in All Phases of Clinical Trials with Oakwood Labs
Our team utilizes a continuous flow process in the scale-up process of your drug – we run the process longer and control the downstream processing so that the product is not changed over the course of the run. Additionally, we validate the scale-up process prior to the manufacture of clinical trial materials, and we use Quality by Design (QbD) principles and develop specific Design of Experiments (DoE) to increase the scale-up success.
From scale-up to Phases 1, 2, and 3, our team has the right combination of knowledge and expertise to guide you through the process of a clinical trial.
Contact Us for Your Phase 3 Clinical Trials
We are proud to be your source for all phases of long-acting injectable (LAI) development. With over two decades of drug encapsulation experience, our team is here to work with you on your proof of concept, feasibility studies, clinical trial material, and FDA-approved commercial supply.
In need of guidance for your Phase 3 clinical trial? Contact our team today!
The goal of clinical trials is to identify whether a designated treatment will benefit an identified population. This process applies to brand-new treatments that develop as well as treatments that were developed with the intention of replacing an older, less effective one.
From start to finish, the process of developing medicine is conducted methodically. Once your formulation has been scaled up and is ready to be tested on the intended human population, it is time to move on in the process to Phase 1 and Phase 2 clinical trials.
Phase 1 Clinical Trials
During a Phase 1 clinical trial, the goal is ultimately to evaluate the safety of a drug. It is often started with a smaller sample of healthy individuals who volunteer to be tested on. This gives insight into how the drug will impact the overall population and sparks research questions for Phase 2 clinical trials.
The Process
In the process of Phase 1 clinical trials, the group is first injected with a small dose of the medicine and monitored. Often, researchers are looking for severe side effects, since this is the first time the drug is being tested on the intended population. It is also common to look for the amount of a drug that is in the blood after administration and the overall effects of the drug on the body.
Researchers may also look for the side effects associated with increasing the dosage of the drug. This is helpful in determining the maximum dosage of the drug that can be administered without side effects. After notes are taken, they are evaluated and researched before moving onto Phase 2 of a clinical trial.
Phase 2 Clinical Trials
In a Phase 2 clinical trial, the goal is to perfect the dosage and test its effectiveness. Ultimately, this equates to the idea of maximizing benefits while minimizing risks to the user. In this step, the medicine is rigorously tested for its success in treating, preventing, or diagnosing a problem or disease.
The Process
During Phase 2 clinical trials, it is typical to test a much larger sample to better understand the drug’s effectiveness. Phase 2 can be thought of as comprising two halves. The first half focuses on dosing, where patients are given different amounts of the drug. It is important to carefully monitor changes when administering different doses. The second half of the trial is reserved for testing the efficacy of the drug.
After these steps are completed, the drug is then evaluated for its benefits and risks. Should the benefits outweigh the risks of the drug, the testing moves on to Phase 3 clinical trials for further assessment.
Choose Oakwood Labs as Your Clinical Trial Destination
Your Phase 1 and 2 clinical trials are critical to providing the public with effective medicine. The testing that needs to be conducted to make sure drugs are safe and effective for patients is extensive. When you discover a new drug that you want to take to market, it is important to be supported by a team that is both knowledgeable and experienced.
Oakwood Labs has the environment and qualified team needed to assist in the development of your treatment. Since 1997, our team has been providing therapeutic benefits to patients through the use of sustained-release pharmaceutical injectables. We operate in a fully FDA-compliant aseptic manufacturing facility to enhance our development capabilities and range of services.
Additionally, we have the ability to help with other projects. We offer the following services, making us the source for supporting all phases of long-acting injectable development:
- Feasibility studies
- Analytical development
- Scale-up capabilities
- GMP clinical trial material
- GMP contract manufacturing
Choose Oakwood Labs as Your Clinical Trial Destination
Oakwood Labs is equipped with the supplies, team, and facilities to help you plan and execute Phase 1 and 2 clinical trials. Contact us today to get started on your next project.