Category Archives: News
Microspheres vs. Microcapsules: Key Takeaways
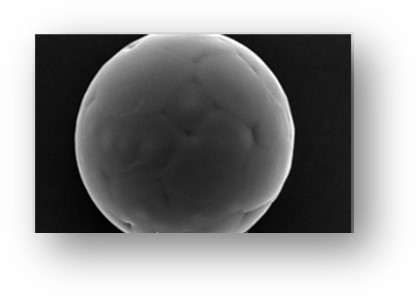
- Microspheres and microcapsules are both microparticles used in sustained-release injectable pharmaceuticals, but they have distinct structures. Microspheres are hollow with a solid outer casing, while microcapsules have a solid or liquid core enclosed in a shell-like coating.
- Microspheres, ranging from 1 μm to 1 mm in size, are made of polymers and can be delivered through various routes, including oral, parenteral, nasal, ophthalmic, and transdermal methods.
- Oakwood Labs has a patented microsphere technology, Chroniject™, which offers several advantages, including rapid formulation development using small-scale batches, easy scalability, compatibility with various molecules, and flexible release durations from one week to one year.
As you know, Oakwood Labs is a global leader in sustained release injectable pharmaceuticals. One of the key components of these drugs are microparticles, which are delivery systems for water-insoluble and sparingly water-soluble agents. Microparticles are split into microspheres and microcapsules.
Both of these play an important role in the pharmaceutical development process at Oakwood Labs. Learn more about microspheres vs. microcapsules.
What Are Microspheres?
Microspheres are small spherical particles that have a solid outer casing, are hollow, and do not contain fluid inside. These particles are made out of polymers and are used in drug delivery applications due to the high surface area, but low particle size. Their size ranges from 1 μm to 1 mm, making them effective for traveling within different areas throughout the body, which helps increase absorption rate and bioavailability.
Microspheres can be delivered through oral, parenteral, nasal, ophthalmic, and transdermal methods. They attach to proteins, peptides, antibodies, antigens and other small molecules to travel through the body and release at controlled rates.
Using microspheres in drug delivery has many benefits including release rates are tailored to specific applications (constant rate of delivery or rapid release), an increase in patient comfort and compliance by reducing the dosing frequency, and more.
What Are Microcapsules?
A microcapsule is also a small spherical particle, but its size is a little different, being 50 nm to 2 mm. The structure of a microcapsule is that of a membrane-enclosed core with a shell-like coating. Sometimes there are multiple cores within the coating. The core is composed of a drug constituent, stabilizers, or additives. The outside coating consists of an inert polymer, coloring agent, plasticizers, resins, waxes and lipids.
Microcapsules allow the core to be isolated from the outside environment and then released when desired. These microcapsules are used for sustained drug release, converting liquid drugs in a free-flowing powder, reducing toxicity and GI irritation, and for drugs that are sensitive to oxygen, moisture or light.
The Key Differences Between Microspheres and Microcapsules
The major difference between microspheres vs. microcapsules is the structure of the particle. As mentioned previously, microspheres have a solid casing and are hollow, while a microcapsule has a solid or liquid core in a shell-like coating.
The main reason for microencapsulation in pharmaceuticals is to protect drugs from environmental hazards like humidity, light, oxygen or heat. They also help controlled release within the patient and avoid too rapid of a release.
Microspheres are manufactured out of different types of polymers that when injected react within the patient to release a certain amount of the drug. While microspheres and microcapsules achieve similar goals for controlled drug release, their structures are what sets them apart.
Our Process for Manufacturing Microsphere Technology
At Oakwood Labs, our microsphere manufacturing capabilities have solved complex pharmaceutical industry challenges for more than 20 years. Our microsphere technology Chroniject™ is a patented, polymer-based injectable microsphere system for drug delivery. This technology is unmatched, and some of its major benefits include:
- Use of small-scale batches allow for rapidly developed formulations
- Formulations are easily scalable
- Process parameters are well-controlled
- Compatibility with peptides, small molecules, and proteins
- Excellent stability
- Flexible release durations ranging from one week to one year
Reach Out Today for More Information on Microcapsules and Microspheres
Our team is fully equipped and operates a staffed research and development facility specifically created to manufacture sustained release injectables. Oakwood Labs is committed to developing products to provide therapeutic benefits to patients.
If you are interested in learning more about microspheres vs. microcapsules for drug delivery, contact us at Oakwood Labs today.
Medications are classified and categorized in many ways including when it comes to distribution methods, dosage, intent, and more. For the purposes of this post, we are going to focus on the rate at which medications are released in the body to serve their intended function.
The time it takes for a drug to run its course will impact all of the characteristics mentioned above, so we’ll look at extended-release medication and modified-release medication and how it applies to drug delivery.
What Is the Difference Between Immediate-Release Drugs and Extended-Release Drugs?
Immediate-Release Drugs
For the purposes of comparison and to fully understand the role of extended-release medications, we’ll start by acknowledging the most common oral dosage for medication, which is immediate release. Any medications classified as immediate release, or IR, go to work very quickly and for a short period of time.
Modified-Release Drugs
As part of an effort to administer medications more conveniently and provide worthwhile options for patients, modified-release medications began appearing on the market. These medications were developed to extend and/or delay their effects to better aid the patient. Their design often means that a patient is required to take medication less frequently, which in many cases is majorly beneficial to their lifestyle.
Modified-release drugs, also known as extended-release drugs or slow-release drugs, are designed to last longer in your body. For example, someone administered an extended-release medication may only need to take one to two doses as opposed to three to four doses of an IR medication. From a physiological standpoint, these medications are absorbed much slower by the patient and do not start breaking down until they reach a certain area of the body.
What Purposes Do Slow-Release Drugs Serve and How Can Oakwood Labs Help?
As mentioned above, modified-release drugs require less frequent doses while still delivering the same medicinal benefits as their counterpart IR medications. This can be quite beneficial when it comes to an individual who has trouble taking medication on a set schedule or more than once a day.
One type of extend-release medication that is frequently used is long-acting injectables. Oakwood Labs is a leader in the development and manufacturing of these extended-release injectables, and some benefits of this type of drug administration include:
- Fewer injections needed over a determined period of time
- Ability to target precise areas of the body
- Improved patient compliance
- Help in preventing drug abuse
Our team has over 20 years of drug encapsulation experience and can work closely with you in developing long-acting injectables for your needs. Visit our homepage to learn about everything we can provide.
Contact Oakwood Labs for Extended-Release Medications
Oakwood Labs has been working in pharmaceutical development for over two decades and has a team of dedicated scientists ready to help your business from concept to finished product.
If you are interested in working with us or would like to learn more about slow-release medications, contact our team today!
Key Takeaways About the Stages of Drug Development
- The pharmaceutical development process is a structured, multi-step procedure involving discovery, preclinical testing, clinical research, FDA review, and post-market monitoring, all aimed at ensuring safety and effectiveness.
- Each pharmaceutical phase serves a distinct purpose, from identifying promising compounds and testing them in controlled environments to confirming results in human trials and evaluating overall effectiveness.
- Regulatory standards play a critical role in the stages of drug development, with the FDA overseeing not only the approval process through requirements like new drug applications but also ongoing monitoring once drugs are on the market.
- Scientific rigor and safety remain central throughout, as researchers evaluate dosage, side effects, interactions, and long-term impact to bring reliable treatments to patients.
Designed to work as a long, persistent, and rigorous process, drug development is crucial to progressing the field of medicine. Drug researchers and developers are tasked with expanding on previous information and findings in order to combat the rise of new, as well as prevalent, diseases.
Because of this, our team at Oakwood Labs wants to highlight the five drug development stages to further encapsulate the rigor of the process of providing sustained-released injectable pharmaceuticals for the community.
A Brief Introduction to Pharmaceutical Phases
As addressed by the FDA, the stages of drug development follow a similar trend for each case. Because of this, we have outlined each drug development stage below, as we feel it is important for our clientele to have an inside look at the processes that we adhere to.
Discovery and Development
Known as the first stage of drug development, the discovery and development stage starts with an initial idea that is fabricated through consistent research and trends. During this stage, scientists discover new insights into the disease process, which allows them to develop a design for a product to either stop or reverse the effects of the disease under consideration. From there, several tests of the molecular compounds are tested to find the possible beneficial effects, or even test existing treatments that might possibly have unanticipated effects.
Once researchers find results of a promising compound through the discovery process, they conduct further experiments to gather information on the following hypotheses:
- How the promising compounds absorb, distribute, metabolize, and dispose of waste
- The mechanisms of action, and how they can be beneficial to further drug development
- The best dosage of the future drug
- The best way to give or take the drug, such as by mouth or as an injectable
- The side effects of the drugs, also known as toxicity
- How it affects individuals of different races, genders, and ethnicities differently
- How it correlates and interacts with other drugs and treatments
- Its overall effectiveness compared to similar treatments
After conducting the series of tests to gauge additional information, researchers are then ready to conduct the preclinical research stage of drug development.
Preclinical Research
As a precautionary measure, researchers must further test their drug for toxicity before distributing the finalized product for public use. Presented in the form of either in vitro or in vivo research, this stage of drug development must be conducted in favor of good laboratory practices (GLP), which can be found in 21 CFR Part 58.1: Good Laboratory Practice for Nonclinical Laboratory Studies. These regulations essentially set the minimum basic requirements for features, such as study conduct, personnel, facilities, equipment, written protocols, and operating procedures, among others.
Though preclinical studies are not usually large, they must provide very stringent and detailed information regarding dosing and overall toxicity levels. Once completed, researchers will assess their findings and decide whether the drug should proceed to the clinical research stage of drug development.
Clinical Research
During this drug development stage, the drug that was initially tested in the preclinical trial will now be tested on people with the hopes of finding how it will interact with the human body. But before the clinical trial can even start, developers will need to design the clinical study for the trial.
When designing a clinical trial, the drug development team follows a specific study plan or protocol that is initially developed by the researcher or manufacturer. Then, the researchers will review the information from the previous two stages to develop research questions, objectives, and decide the following:
- A selection criterion of who qualifies to participate
- The number of people allotted for the study
- The duration of the study
- If there should be the presence of a control group to limit research bias
- How and at what dosage the drugs will be administered
- What assessments will be conducted and what data will be collected
- How the collected data will be reviewed and analyzed for future drug development stages
Unlike the preclinical trials, the clinical trials range from early, small-scale studies to late-stage, large-scale studies.
FDA Review
Considered the final drug development stage, the FDA review team will thoroughly examine the evidence from the drug’s early tests, preclinical and clinical research, and decide to either approve or not to approve the product.
During this stage, a New Drug Application (NDA) must be provided to outline the entirety of the drug development stages that lead to the anticipated approval. The NDA must include the following when presented:
- Proposed labeling
- Safety updates
- Drug abuse information
- Patent information
- Any data of studies that were not conducted within the United States
- Institutional review board compliance information
- Clear and concise directions for use
Finally, the drug is sent for approval to the FDA, where the committee decides whether the drug is safe and effective for public use. The process of approval can take anywhere from 6 to 10 months, as it goes through several inspectors, project managers, and pharmacologists.
From there, the drug is passed to the labeling and marketing stage, where the remaining issues will need to be resolved, and labeling will be provided accurately and objectively for public discretion.
FDA Post-Market Safety Monitoring
Though there are rigorous and persistent steps during each stage of drug development up to its approval and distribution, limitations are still evident and existent. Because of this, the FDA closely monitors drugs for months and even years so they can review the reports on the prescription and decide whether or not to add cautions to the dosage or usage information, which can still be seen as part of the pharmaceutical development process.
If there were significant changes that needed to be made to a product after the drug development stages were complete, developers would have to file a supplemental application in order to make those changes.
Why Choose Us for Your Pharmaceutical Needs
At Oakwood Labs, our drug development team is honored to provide sustained-released injectables to our extensive clientele. On top of that, some of the benefits of working with our pharmaceutical company through each stage of drug development include the following:
- Pre-formulation development
- Feasibility studies
- Scaling formulation
- Toxicology batch manufacturing
- ICH compliant stability tests
- Small-scale to large-scale clinical trial manufacturing
- Commercial and aseptic GMP manufacturing
Contact Oakwood Labs to Learn More About the Stages of Drug Development
If you have any specific questions regarding the drug development stages that Oakwood Labs conducts for sustained-release pharmaceuticals, please contact our team today. We look forward to assisting you in any way we can.
In order for injectables to be able to successfully provide sustained releases, they necessitate drug-encapsulating devices. For over 50 years, encapsulation has been one of the many techniques used in the pharmaceutical industry. In this process, medication is released from a microsphere by the drug detaching from the polymer or through the degradation of the polymer matrix.
Keep reading to learn more about the role of polymer microspheres in the process of drug delivery and how our technology can be an asset to your long-acting injectable project.
The Use of Polymer Microspheres in the Pharmaceutical Industry
Polymer microspheres are often used in drug delivery because they have a high surface area and low particle size, which increases their absorption rate and bioavailability. Since these microspheres are small in size, they are also efficient when moving throughout the body. They are additionally capable of sustained release, making them ideal for use in long-acting injectables for both passive and active targeting.
Capabilities of Microspheres
Microspheres can be organic or inorganic and are designed to encapsulate bioactive molecules and release them in a controlled way. They are used for desired release profiles and can be used to target specific delivery sites throughout the body, including organs.
Additionally, they are considered rigid which means that they can be packed together, found alone, or even combined with other biomaterials to create porous 3D-structured scaffolds. In these scaffolds, they can either serve as a component of a larger scaffold or be the building blocks of one.
Factoring in the Polymer
Polymers can take the role of bioactive or biodegradable depending on the agents they are combined with. When incorporated with therapeutics, polymers become bioactive and provide their own therapeutic benefit. However, they can also be biodegradable which improves release kinetics and helps prevent carrier accumulation.
The benefits of using polymers for encapsulation include the following:
- Ability to encapsulate a variety of drugs
- They have high biocompatibility
- They are bioavailable
Incorporating ChronijectTM Technology into Your Project
Since polymer microspheres are suited for sustained-release technology, they are the perfect candidate for long-acting injectables. Our technology for microspheres is a patented, polymer-based injectable for drug delivery called ChronijectTM. This technology is compatible with small molecules, peptides, and proteins. It is also capable of enabling sustained release over durations of two weeks to one year.
Our ChronijectTM process for microsphere manufacturing initially uses small batch sizes to enable the production of numerous trial batches. This allows us to obtain a formation with the desired release profile and other indicated product characteristics. Additionally, this process can easily be scaled up to commercial production levels with lot-to-lot reproducibility. Since we can produce clinical trial material at the forecast commercial scale, scaling up is feasible at the commercial scale and is conducted in our GMP facility.
Advantages of Using ChronijectTM
There are diverse advantages of using polymer microspheres and our ChronijectTM technology for drug delivery, which include the following:
- Rapid development of formulations using small-scale batches
- Easily scaled formations
- Process parameters that are monitored and controlled
- Proven lot-to-lot reproducibility
- Molecule compatibility (small molecules, peptides, proteins)
- Flexible release durations, ranging from one week to one year
- Excellent stability
- Instant reconstitution with WFI – no special diluent required
- Applications in various therapeutic indications (CNS, neurology, ophthalmology)
About Oakwood Labs
Oakwood Labs is a specialty pharmaceutical company located near Cleveland, Ohio. We are focused on developing and manufacturing sustained-release injectable pharmaceuticals, incorporating our microsphere-based technology. We operate in a fully compliant, aseptic facility that is specifically used for manufacturing pharmaceutical products like injectables.
Our goal is to provide enhanced therapeutic benefits to patients while achieving excellent returns for our pharmaceutical partners. With over 20 years of experience, we have proven ourselves as an effective partner of global pharmaceutical firms. From proof of concept and feasibility studies to clinical trials and FDA-approved supplies, the team at Oakwood Labs is ready to help incorporate polymer microspheres into the development of long-acting injectables.
Contact Our Team with Any Questions
If you are interested in learning more about our capabilities in incorporating polymer microspheres into long-acting injectables, please reach out to our team today.
What Are Peptides?
Peptides are strings of amino acids, also known as the “building blocks” of proteins. The human body naturally produces more than 7,000 known peptide types. Along with the peptides that are naturally produced by the human body, they can also be produced in medical laboratories. Pharmaceutical peptides are those that are created using recombinant DNA technology which involves manipulating and isolating DNA segments.
Peptides in pharmaceuticals are also often referred to as therapeutic peptides. They are most commonly used for the treatment of diseases. Some examples of lab-produced peptides are human insulin and growth hormone, both of which can be life-saving for people living with certain conditions.
Getting More Specific: What Are the Benefits of Peptides in Pharmaceuticals?
The development of peptides in pharmaceuticals has made a lot of progress in the last decade thanks to new production, modification, and DNA technologies. Some of the most notable benefits of developing and utilizing pharmaceutical peptides include:
- Low production costs and sale prices
- Oral administration allows for easy delivery to patients
- Good membrane penetration ability
There aren’t many cons when it comes to utilizing pharmaceutical peptides in the medial field, but one that should be noted is they tend to be short lived in the body as a result of rapid degradation and clearance.
Our Capabilities in Pharmaceutical Peptide Production
Oakwood Labs has decades of experience in researching and manufacturing a wide range of pharmaceuticals, including pharmaceutical peptides. Our international reputation for pharmaceutical development is all thanks to our highly skilled, innovative, and knowledgeable staff.
When it comes to utilizing peptides in pharmaceuticals, our team is highly equipped to perform research and lead in the development of individually formulated products for your specific needs.
Oakwood Labs Is an Industry Leader
In addition to manufacturing pharmaceutical peptides, which, as we’ve highlighted, are crucial in the management of chronic diseases, Oakwood Labs provides many other products and services that cater to the medical field. Some of those services include:
When you work with Oakwood Labs, you can expect high-quality results every time. We maintain rigorous quality assurance and validation practices to provide the absolute best solutions for our customers. The partnerships we’ve long maintained are attributed to our skilled team and history of compliance.
Reach Out to Us for Pharmaceutical Peptides and More
Oakwood Labs is your source for R&D formulation development through GMP commercial manufacturing. If you’re interested in learning more about our capabilities when it comes to peptides in pharmaceuticals, contact our team today.
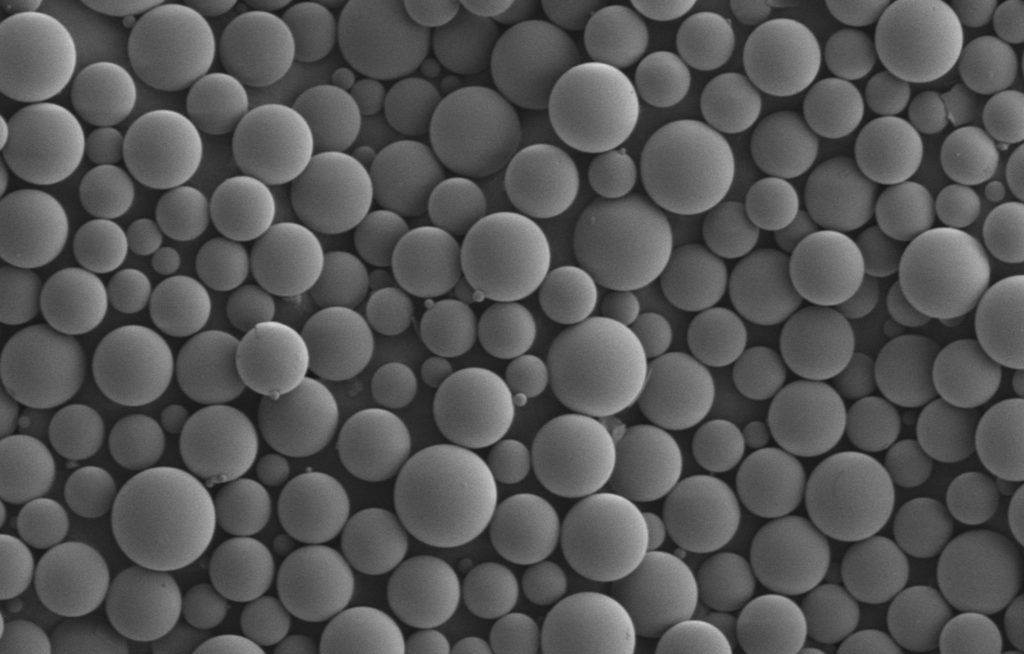
The utilization of biodegradable polymers has come a long way during the past few decades. As science and engineering continue to evolve, so do the advancements with utilizing biodegradable polymers in drug delivery. Let’s examine some key aspects.
Starting with the Basics: What Are Biodegradable Polymers?
A biodegradable polymer is engineered to deteriorate over time. These specific polymers have a high molecular weight that depreciates to a lower molecular weight after its intended function. Essentially, once these polymers have served their purpose with appropriate response from the host, they are broken down and eliminated from the body.
Biodegradable polymers are produced both naturally and synthetically. The two most common natural biodegradable polymers include proteins and polysaccharides. Synthetic biodegradable polymers consist of amides, ethers, urethanes, and other esters.
PLA and PLGA are two common biocompatible and biodegradable polymers. These FDA-approved polymers have been used in medical devices, such as microspheres. One of the major benefits of working with biodegradable polymers, such as PLA and PLGA, is that they can be used to encapsulate just about any type of drug, including:
- Proteins
- Peptides
- Polypeptides
- Small molecules
In addition to using biodegradable polymers in controlled drug delivery, they can also be used for the following applications:
- Gene delivery
- Wound dressings
- Tissue regeneration and engineering
- Cryopreservation
- Enzyme immobilization
- Nanotechnology
- Orthopedic fixation devices
- Prosthetics
- Biomedical devices and implants
- Cosmetics
- Augmentation
- Stitches
Why Are Biodegradable Polymers Effective?
Whether biodegradable polymers are natural or synthetic, they have unique biocompatible properties that allow them to decompose inside the body by natural, nontoxic byproducts such as carbon dioxide or water. One benefit to synthetic biodegradable polymers is that they can be produced with a wide range of mechanical, physical, and chemical properties that can be modified. Because these specific polymers can be synthesized by reproducing the same polymer, they have endless availability.
Naturally occurring biodegradable polymers are just as effective as synthetic in that they offer temporary support and aid in controlled release factors throughout the body. Some other major advantages of using biodegradable polymers in drug delivery include:
- Ability to adjust the degradation rates and mechanical properties to form into various configurations
- Decreased need for successive surgical removal, which overall saves money and time
- Sterilizable properties that can be easily processed by the host
- Modifiable surfaces
Biodegradable Polymers at Oakwood Labs
Oakwood Labs is a leader in the pharmaceutical industry providing manufacturing services and contract development. Our state-of-the-art technology allows us to be experts with biodegradable polymers in drug delivery.
At Oakwood labs, we utilize biodegradable polymers such as PLGA, PLA, and other polymers that demonstrate low toxicity. These biodegradable polymers are used in our long acting injectables, which are based upon our technology platform Chroniject™. Our patented microsphere technology is a polymer-based injectable microsphere system for drug delivery.
Other advantages of our technology used for biodegradable polymers in drug delivery include:
- Sourced from multiple suppliers
- Quick and effective development of formulations using small scale batches
- Easily scalable formulations
- Controlled process parameters
- Proven lot-to-lot reproducibility
- Molecule compatibility (peptides, proteins, and small molecules)
- Adjustable release durations ranging from one week to one year
- Exceptional stability
- Immediate reconstitution with WFI with no special diluent needed
- Versatile use in multiple therapeutic applications (neurology, depression, CNS, ophthalmology)
Contact Us Regarding Our Biodegradable Polymers in Drug Delivery
At Oakwood Labs, we have over 20 years of experience with drug encapsulation, which has propelled us to being a leader in sustained release drug delivery. When it comes to using biodegradable polymers in drug delivery, our advanced technology provides the ability to customize release duration ranging from weeks to years.
If you’re interested in learning more about biodegradable polymers, reach out to our team today.
When caring for patients, it is critical to use medical treatments that are not only effective but also seamlessly incorporate into their lifestyles. With the risks associated with pills and the need for a regular routine, medical personnel often will turn to long-acting injectable antipsychotics for patient treatment due to the approach’s benefits.
Long-acting injectable antipsychotic drugs are commonly used for patients who experience unpleasant adverse side effects of antipsychotic drugs and are hesitant to believe they have a mental illness. While this form of medicine does not require the daily administration that other antipsychotics do, they are able produce relief to patients in need.
Administering Long-Acting Injectable Antipsychotics
Long-acting injectable antipsychotics can be utilized with diverse medications, making them ideal for patients suffering from an assortment of issues. Along with this, long-acting injectable antipsychotics are often administered in two-, three- or four-week intervals, but treatments can vary based on the patient’s needs. Time between injections also differs based upon the administered drug and its ability to stay within the system.
Candidates for this treatment often include patients with a history of non-adherence, severe symptoms, comorbid substance abuse, and cognitive impairment. Additionally, this method is extremely beneficial to patients who experience ambivalence or negative attitudes toward medications.
Why Long-Acting Injectable Antipsychotics Are Ideal for Patients
There are many advantages to using long-acting injectable antipsychotics on eligible individuals, as they can improve the lives and routines of patients in the following ways:
- Reduced accountability – In order for medications to work best, it is important for patients to take them every day according to instructions. Since long-acting injectable antipsychotics stay in the system for extended periods of time, they reduce the need for a daily reminder to take the drug.
- Regularity – By using long-acting injectable antipsychotics, the need for medical appointments increases. This creates regular interactions between the patient and medical staff, creating a more comfortable environment, providing more social interactions, and solidifying a routine.
- Stability – The long-acting injectable method treats patients with more stable plasma concentrations than oral medications. It also lessens the frequency of having peak plasma levels, which can result in fewer side effects for the patient. Lastly, it reduces relapse frequency and rehospitalization rates, making it a great alternative for those who are prone to hospital visits already.
- Safety – Since long-acting injectable antipsychotic drugs are administered by medical personnel, they are safer for patients. The approach reduces the risk of accidental or deliberate overdose through the use of regulated injections that work gradually over time.
Oakwood Labs Is Ready to Help You Produce Long-Acting Injectable Antipsychotics and More
Oakwood Labs is a leading manufacturer of sustained-release pharmaceuticals with over 20 years of drug encapsulation experience. We offer support throughout the phases of long-acting injectable development, ultimately achieving a product that provides enhanced therapeutic benefits to patients everywhere.
In our facilities, we can provide the following to help achieve your project goals, including those involving long-acting injectable antipsychotics:
- Pre-formulation development
- Feasibility studies
- Scaling formulation
- Toxicology batch manufacturing
- ICH compliant stability tests
- Phase I, II, and III clinical trial manufacturing
- GMP manufacturing (commercial and aseptic)
Contact Us Today to Learn More!
Long-acting injectable antipsychotics are a way to steadily prevent the intense symptoms associated with mental illnesses. Using this approach has the ability to put patients on the path to a stable and successful life.
The team at Oakwood Labs is ready to help with the development of your long-acting injectable antipsychotic drugs. Please reach out today and get started on your project with us!
The goal of clinical trials is to identify whether a designated treatment will benefit an identified population. This process applies to brand-new treatments that develop as well as treatments that were developed with the intention of replacing an older, less effective one.
From start to finish, the process of developing medicine is conducted methodically. Once your formulation has been scaled up and is ready to be tested on the intended human population, it is time to move on in the process to Phase 1 and Phase 2 clinical trials.
Phase 1 Clinical Trials
During a Phase 1 clinical trial, the goal is ultimately to evaluate the safety of a drug. It is often started with a smaller sample of healthy individuals who volunteer to be tested on. This gives insight into how the drug will impact the overall population and sparks research questions for Phase 2 clinical trials.
The Process
In the process of Phase 1 clinical trials, the group is first injected with a small dose of the medicine and monitored. Often, researchers are looking for severe side effects, since this is the first time the drug is being tested on the intended population. It is also common to look for the amount of a drug that is in the blood after administration and the overall effects of the drug on the body.
Researchers may also look for the side effects associated with increasing the dosage of the drug. This is helpful in determining the maximum dosage of the drug that can be administered without side effects. After notes are taken, they are evaluated and researched before moving onto Phase 2 of a clinical trial.
Phase 2 Clinical Trials
In a Phase 2 clinical trial, the goal is to perfect the dosage and test its effectiveness. Ultimately, this equates to the idea of maximizing benefits while minimizing risks to the user. In this step, the medicine is rigorously tested for its success in treating, preventing, or diagnosing a problem or disease.
The Process
During Phase 2 clinical trials, it is typical to test a much larger sample to better understand the drug’s effectiveness. Phase 2 can be thought of as comprising two halves. The first half focuses on dosing, where patients are given different amounts of the drug. It is important to carefully monitor changes when administering different doses. The second half of the trial is reserved for testing the efficacy of the drug.
After these steps are completed, the drug is then evaluated for its benefits and risks. Should the benefits outweigh the risks of the drug, the testing moves on to Phase 3 clinical trials for further assessment.
Choose Oakwood Labs as Your Clinical Trial Destination
Your Phase 1 and 2 clinical trials are critical to providing the public with effective medicine. The testing that needs to be conducted to make sure drugs are safe and effective for patients is extensive. When you discover a new drug that you want to take to market, it is important to be supported by a team that is both knowledgeable and experienced.
Oakwood Labs has the environment and qualified team needed to assist in the development of your treatment. Since 1997, our team has been providing therapeutic benefits to patients through the use of sustained-release pharmaceutical injectables. We operate in a fully FDA-compliant aseptic manufacturing facility to enhance our development capabilities and range of services.
Additionally, we have the ability to help with other projects. We offer the following services, making us the source for supporting all phases of long-acting injectable development:
- Feasibility studies
- Analytical development
- Scale-up capabilities
- GMP clinical trial material
- GMP contract manufacturing
Choose Oakwood Labs as Your Clinical Trial Destination
Oakwood Labs is equipped with the supplies, team, and facilities to help you plan and execute Phase 1 and 2 clinical trials. Contact us today to get started on your next project.
With over 20 years of experience developing time-release pharmaceuticals, Oakwood Labs is a trusted industry source for pharmaceutical products that include PLA and PLGA microspheres. PLGA and PLA polymers are FDA-approved biodegradable and biocompatible polymers that have been used in medical devices, including microspheres.
PLA Microspheres vs. PLGA Microspheres
PLA and PLGA microspheres are widely studied polymers in the medical field, as they allow for the encapsulation of the desired active pharmaceutical ingredient or drug. The PLA microspheres aide in controlling the release of the drug from the polymer matrix and allow for a sustained release of the drug over a period of time that can vary from weeks to several months.
PLA microspheres exhibit asymmetric centers in their backbone which result in either D or L forms, producing PDLA or PLLA. PLGA is the copolymer of D, L-lactic acid with glycolic acid.
While both PLA and PLGA microspheres are insoluble in water, their absorption of water causes them to degrade over time. The methyl groups of PLA microspheres decrease the water uptake properties of the polymer, thereby extending its time-release properties.
One of the most important factors that affects the degradation rate of PLA and PLGA microspheres is the molecular weight of the polymer. Crystalline parts of the polymer exhibit more resistance to degradation. As Keles states, “the crystallinity of the polymer depends on compositions. For example, increasing the percentage of glycolide monomer in PLGA backbones decrease polymer crystallinity.”
PLA and PLGA Microspheres Play a Critical Role
One advantage of working with PLGA and PLA microspheres is the fact that the polymers can be used to encapsulate almost all types of drugs. This includes:
- Small molecules
- Peptides
- Polypeptides
- Proteins
It’s possible for the microspheres to have such diverse abilities because they come in a variety of comonomer ratios, molecular weights, and end-capping configurations. This allows the release duration to be tailored for each specific drug. In addition, the release profile, such as the burst release, can be modified by choosing the appropriate polymer for the reaction.
The release profile of the drug over time is dependent on the specific drug that is being encapsulated by the PLA and PLGA microspheres. Interactions between the polymers and the drug can be positive or adverse and require an in-depth understanding to develop a successful microsphere product.
Our Process of Developing PLGA and PLA Microspheres
In initial production stages, we utilize small batches to allow for testing of a multitude of trial batches and rapid production. As we develop the formulation with the desired time-release profile and additional desired characteristics, we then refine methods of production. Our processing of developing PLA and PLGA microspheres starts in small batches to ensure that all desired properties are met and can then be scaled to a commercial production process.
Our Chroniject™ PLA/PLGA microsphere time-release injections are a leader in the industry and have several key benefits. Some of the benefits include:
- Rapid development of formulations with small-scale batches that are easily scalable
- Proven lot-to-lot reproducibility
- Flexible release durations that vary from one week to one year
- Applications in multiple therapeutic indications (CNS, neurology, ophthalmology)
Contact Us for Your PLA/PLGA Microsphere Needs
Oakwood Labs is equipped to support all development phases of time-release drugs using our PLA/PLGA microsphere technology. If you are interested in knowing more about how our microspheres can help you, contact our team today.
This year’s PDA Annual Meeting is occurring April 4-6 in Dallas, TX. This event is focused on the theme of “Level Up: Agility in the New Normal” and will highlight items to look forward to in the future of pharmaceutical manufacturing.
Incorporating speakers, networking events, and panel discussions, this is an event that you will not want to miss. This year, we are excited to announce that we will be featured as an exhibitor.
We Look Forward to Seeing You
Our team has been focused on researching and developing sustained-release injectables, and we are always looking for opportunities to chat about our discoveries and processes. If you are looking for an aseptic lab staffed by experts, consider Oakwood Labs for your next project, as we can assist you in the following:
- Pre-formulation development activities
- Feasibility studies
- Scale-up of formulation
- Manufacturing toxicology batches
- ICH compliant stability testing
- Clinical trial manufacturing (Phase I, II, III)
- Commercial aseptic GMP manufacturing
We are so excited to meet you and show you what we have been working on, so be sure to come to visit the Oakwood Labs team at Booth #308.
Don’t Hesitate to Reach Out
The 2022 PDA Annual Meeting is sure to inform us about new trends and inspire us to be innovative as we take on the pharmaceutical challenges that lie ahead. If you are unable to attend the event, we would be happy to chat about our experience at the occasion or provide information on extended-release technology and more.
Reach out today with any questions you may have. We look forward to seeing you soon!