Archives by Day: February 27, 2024
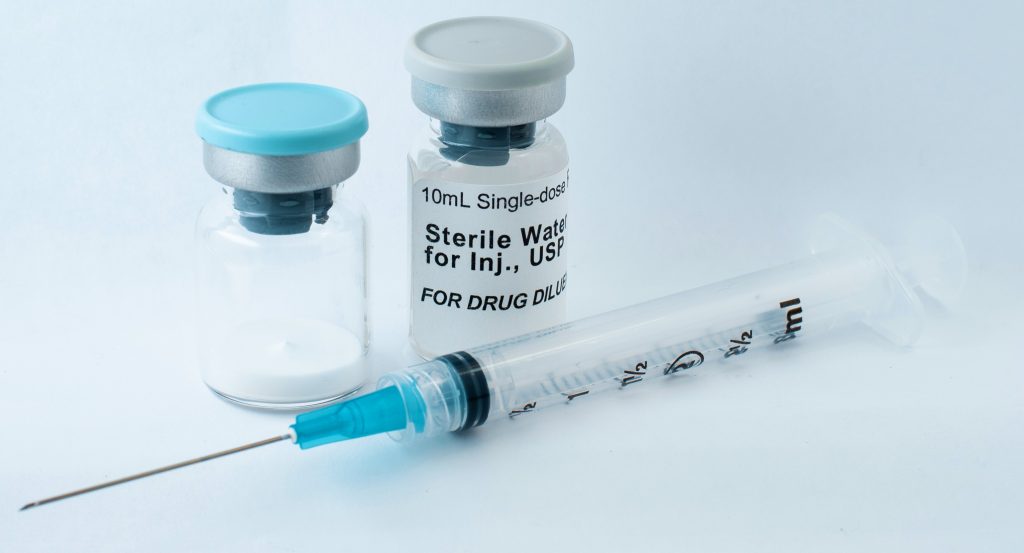
Oakwood Labs is a global leader in sustained-release drug delivery. Our facility provides feasibility studies, analytical development, scale-up capabilities, GMP clinical trial material, GMP contract manufacturing, and sterile fill-finish.
Sterile fill-finish is the final leg of the pharmaceutical manufacturing process, where a substance is filled into vials, ampoules, or other containers deemed appropriate. After the substance is put into containers, it is ready to be sent out for distribution. Learn more about our capabilities for this part of the process below.
The Process for Fill-Finish Pharmaceuticals
There are a variety of reasons that sterile fill-finish is essential in the manufacturing of long-acting injectables. Patient safety is at the top of the list, however, as the approach ensures that the product is free from microbial contamination.
What the Process Entails
When you begin the sterile fill-finish step, you are almost at the end of the drug manufacturing process. However, this is a critical point of the overall manufacturing process because it needs to be done correctly to ensure that your drug is safe for patients. At this point, you’ll want to request help from a fill-finish CDMO who can oversee the process properly.
Some of the steps that will be completed will include:
- Filling – In the filling process, the drug is placed into the final product containers under controlled and sterile conditions. Finish and fill services must be completed in a sterile environment to prevent contamination. For this reason, these services are performed in a cleanroom that has controlled air quality, temperature, and humidity. Additionally, it is important to ensure that sterile filling techniques are also applied to equipment and packaging.
- Container closure – It is important to select the appropriate container closer that is going to maintain the sterility and stability of the drug. Whether this is a vial, syringe, ampoule, or something else, the team you work with will help find the right solution for your application.
- Quality control – In the process of fill-finish for pharmaceuticals, rigorous quality control is practiced to ensure that the product meets regulations. Not only is the product tested throughout the process, but it also undergoes environmental monitoring and final product review to ensure that it is sterile and potent, and that it meets all other criteria that is required.
- Labeling and packaging – Once the product is filled, the finished item is labeled and packaged according to regulations and any requirements provided by outside sources.
- Regulation compliance check – It is important for aseptic, CDMO facilities to adhere to regulatory guidelines like Good Manufacturing Practice (GMP). This helps to ensure safety, efficacy, and quality, completing the final steps of the finish and fill process.
When you choose to work with a fill-finish CDMO, you can also receive different batch sizes to meet your project’s needs. From small-scale clinical trials to large-scale commercial production, our processes can be adapted to your specifications.
Let Oakwood Labs Be Your Fill-Finish CDMO
Oakwood Labs operates a fully compliant aseptic GMP facility. Our FDA-approved facility is headquartered near Cleveland, Ohio and centers on the development and manufacturing of sustained-release injectables for the pharmaceutical industry. We focus on creating pharmaceutical products that provide therapeutic benefits to our patients and excellent returns for our partners.
Whether you need assistance with fill-finish pharmaceutical services or something else, we are here to help. Other services we provide include:
- Formulation and analytical development activities
- Accelerated and long-term stability studies
- Scale-up, validation, or engineering batches
- Complete method transfer and validation
- Batch record and protocol development
FAQs About Sterile Fill-Finish Services
How does Oakwood Labs stand out from other fill-finish CDMOs?
Our company differentiates itself through a combination of experience, capabilities, and a partnership-driven approach designed to support drug development programs at every stage.
Key areas that set our sterile fill-finish and other services apart include:
- Specialized experience – Decades of industry experience supporting more than 100 long-acting injectable projects
- Regulatory and quality expertise – Comprehensive CMC and regulatory support, along with ICH-compliant stability testing
- End-to-end support – Continuity across development and manufacturing stages that helps maintain consistency throughout the product lifestyle
- Collaborative partnership – A hands-on, collaborative approach that prioritizes clear communication and solutions tailored to each program’s needs
What is the difference between aseptic fill-finish and terminal sterilization?
Aseptic fill-finish and terminal sterilization are two methods used to ensure sterile injectable drug products and the appropriate approach meet formulation and product requirements.
Aseptic fill-finish involves sterilizing individual components such as the drug substance, containers, and closures before filling them together in a controlled sterile environment. This method is commonly used for products that cannot radiate heat or radiation.
On the other hand, terminal sterilization takes place after the product has been filled and sealed in its final container. The finished product is then sterilized using heat, making it suitable only for formulations that can withstand these conditions.
What industries commonly benefit from finish and fill services?
Fill-finish services are commonly used across industries that require sterile, injectable drug products, including:
- Pharmaceutical manufacturers
- Biotechnology companies
- Clinical research and development organizations
- Drug development and research firms
- Specialty and injectable therapeutics manufacturers
- Emerging and virtual pharma companies
Consult Our Finish and Fill Services Today!
If you are in need of pharmaceutical manufacturing, partner with Oakwood Labs to receive reliable and efficient services.
Contact us today to learn how you can achieve a sterile fill-finish with our CDMO.

