Our Microsphere Manufacturing Technology
Our Microsphere Manufacturing Technology
Our Expertise in Sustained-Release Injectable Therapies
With over twenty-five years of experience , Oakwood Labs is a trusted leader in the development and manufacturing of sustained-release and targeted-release injectable drug products. Our deep expertise spans the full lifecycle of long-acting formulations, from early-stage research and formulation development to clinical and commercial manufacturing.
At the heart of our capabilities is our proprietary Chroniject™ technology — a patented polymer-based microsphere system designed for controlled drug delivery. This versatile technology is compatible with a wide range of therapeutic molecules, including small molecules, peptides, and proteins, allowing us to address diverse medical needs across multiple therapeutic areas.
Chroniject™ enables precise, sustained release of active pharmaceutical ingredients over durations ranging from one week to one year, helping improve patient compliance, treatment outcomes, and overall therapy effectiveness. By carefully controlling the microsphere composition, particle size, and formulation parameters, Oakwood Labs can tailor drug release profiles to meet the specific requirements of each program.
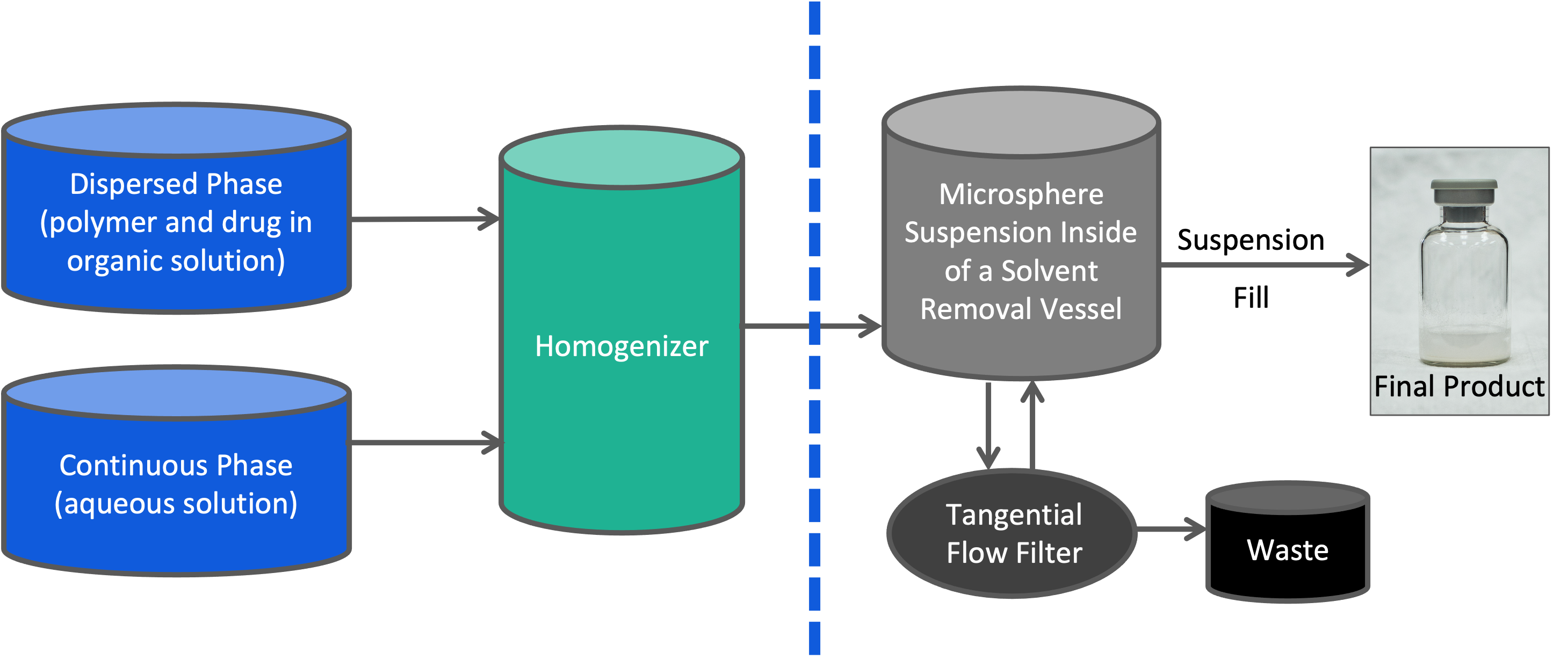
Our Process for Manufacturing Microspheres
Oakwood’s microsphere manufacturing process can be scaled up to commercial production with demonstrated lot-to-lot reproducibility under aseptic conditions. We initially utilize small batch sizes to enable rapid formulation development and testing of numerous trial batches to obtain a formulation with the desired release profile, as well as other product characteristics. In parallel, we develop and refine the analytical methods to test prototype formulations.
Once several promising formulations are tested in vitro, animal studies are performed to determine if the in vitro testing is reflective of the in vivo release profile, and to select a formulation to scale up for human testing. A confirmatory animal study may be conducted with the scaled-up material manufactured in our GMP facility. Clinical trial material is usually produced at the forecasted commercial scale, so that upon success of the clinical trial, scaling up will not be an issue.
To learn more about our microsphere process, check out our formulation development page.
Advantages of Our Microsphere Technology: Chroniject™
As a leading manufacturer of injectable microspheres, Oakwood Labs provides innovative technology via our Chroniject™. When it comes to microsphere systems for drug delivery, Chroniject™ delivers many advantages to your operations.
Some of the key benefits include:
- Rapid development of formulations using small-scale batches
- Formulations are easily scalable
- Well-controlled process parameters
- Proven lot-to-lot reproducibility
- Molecule compatibility (small molecules, peptides, proteins)
- Flexible release durations from one week to one year
- Excellent stability
- Instant reconstitution with WFI – no special diluent required
- Applications in multiple therapeutic indications (CNS, neurology, ophthalmology)
Our Microsphere Analytical Capabilities
At Oakwood Labs, we offer a full range of analytical testing capabilities to support formulation development and clinical supply manufacturing. All of our analytical testing capabilities are able to be completed in-house by our team, allowing for rapid results turnaround and close interactions between the formulation and analytical teams.
The full range of analytical testing capabilities we provide are listed below:
- HPLC
- UHPLC
- GC
- GPC
- In vitro dissolution
- Laser diffraction particle sizing
- DSC
- UV/Vis
- Karl Fischer
- LC/MS (external)
- GC/MS (external)
- NMR (external)
FAQs on Microsphere Technology
What are the reasons for developing a long-acting injectable using microspheres?
Microspheres can be tailored to a specific API and application, leading to better bioavailability, stability, and pharmacokinetics. Additionally, their use can lead to improved intellectual property and life-cycle management opportunities. Choosing long-acting injectables offers the potential for targeted drug release, lower dosage requirements, and improved patient compliance.
Can you alter the polymer in microspheres?
Yes, our process is very customizable. There are a variety of polymer types that we can choose from with varying molecular weights. Typically, we focus on PLGA and PLA polymers, but we have also used co-polymers with PEG, Caprolactone, and even proprietary polymers.
What are the benefits of long-acting injectables?
Long-acting injectables can extend exclusivity of products nearing the end of patent life. In addition to this, other benefits of long-acting injectables include:
- Reducing the number of injections needed
- Improving patient compliance
- Helping prevent drug abuse
- Targeting specific anatomical areas
What kinds of APIs are compatible with microsphere technology?
Large and small molecules, such as peptides, proteins and nucleic acids, have been encapsulated in polymeric microspheres. Biologics are also able to be encapsulated in microspheres that are made of biocompatible and biodegradable polymers, which offers controlled drug delivery.
How do you make microspheres?
Oakwood Labs produces a single or double emulsion utilizing a continuous manufacturing process. This gives us the ability to scale up and manufacture under aseptic conditions. Downstream, we remove the solvents with continued washing through a tangential flow filter. The final product composition consists of an aseptic lyophilized product and excipients in a vial, therefore needing reconstitution with WFI only.
Reach Out to Oakwood Labs for Your Microsphere Manufacturing Needs!
As a global leader in sustained drug release delivery, Oakwood Labs is able to support all phases of long-acting injectable development. Our technology and manufacturing capabilities have been solving complex challenges in the pharmaceutical injectable industry for over 20 years.
If you are interested in injectable microspheres or microsphere systems for drug delivery, contact our team at Oakwood Labs today. We also offer a variety of additional services to help meet your pharmaceutical needs.
Additional Content on Microspheres
- What Are the Differences Between Microspheres and Microcapsules?
- The Role of Polymer Microspheres in Drug Delivery
- PLA/PLGA Microspheres and Their Role in Sustained Release Drug Delivery