Pharmaceutical Formulation Optimization and Scale-Up
Pharmaceutical Formulation Optimization and Scale-Up
The Scale-up Process at a Glance
- Oakwood Labs employs a quality-driven approach to scale-up, focusing on maintaining critical product attributes such as particle size distribution and in vitro release profile from small-scale (5-gram) to large-scale (1-kilogram) batches.
- We utilize a continuous flow process for scale-up, using the same equipment for prototype formulations and full-scale lots. This approach allows for scale-up by running the process longer and controlling downstream processing, with demonstrated success in scaling up to batch sizes of 1, 2, and 8 kg.
- Oakwood Labs implements Quality by Design (QbD) principles and Design of Experiments (DoE) to optimize the scale-up process. We validate the process before manufacturing clinical trial materials and provide necessary CMC documentation for regulatory filings, ensuring compliance with regulatory authorities.
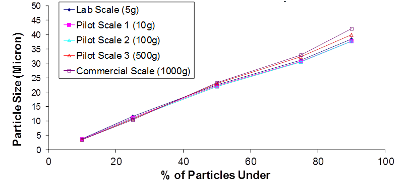
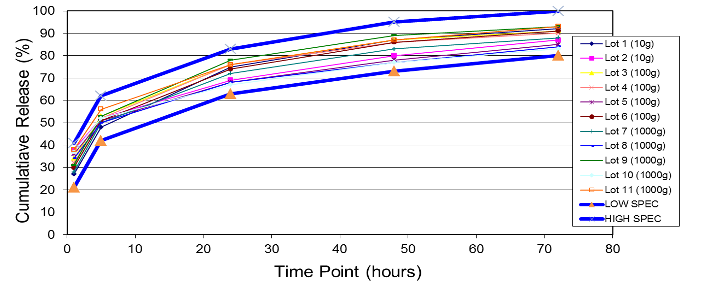
At Oakwood Labs, we use a quality-driven approach to quickly complete the feasibility study and move on to the scale-up process. Our typical approach to the scale-up process is that once a target formulation is selected during the feasibility study and confirmed with an in vivo animal study, the next step is scaling up the formulation while achieving the same critical product attributes. The charts below show two of the critical product attributes that Oakwood focuses on maintaining during the scale-up process.
The first chart is an example of a particle size distribution maintained from a 5-gram batch size through a 1-kilogram batch size. The second chart is an example of achieving a tight but differentiable in vitro release profile. Through the use of our technology we have demonstrated control over these attributes, which leads to a successful pharmaceutical scale-up of the product.
Our scale-up and process controls show proven scalability that start from lab scale and reach all the way to commercial batch sizes. Throughout different phases of scale-up, Oakwood’s process shows consistent lot-to-lot reproducibility.
Example of Particle Size Distribution throughout Scale-Up:
Example of In Vitro Release throughout Scale-Up:
Advantages of the Oakwood Pharmaceutical Scale-Up Process
A key advantage of the Oakwood pharmaceutical scale-up process is that it is a continuous flow process. The equipment used to manufacture prototype formulations is the same as is used to manufacture full scale lots. The scale-up process is accomplished by running the process longer, and by controlling the downstream processing, such that the product is not changed over the course of the run. We have demonstrated scale-up of prototype formulations to batch sizes of 1, 2, and 8 kg.
 We validate our scale-up process prior to manufacture of clinical trial materials, and set specifications of process parameters such as flow rates, mixing speeds, hold times, etc. We use Quality by Design (QbD) principles and develop specific Design of Experiments (DoE) to increase our success of scale-up.
We validate our scale-up process prior to manufacture of clinical trial materials, and set specifications of process parameters such as flow rates, mixing speeds, hold times, etc. We use Quality by Design (QbD) principles and develop specific Design of Experiments (DoE) to increase our success of scale-up.
For setting many of the specifications of process parameters, we consider small batches to be another advantage of the Oakwood scale-up process. Small batches can be made to determine ranges of the process parameters, instead of needing to manufacture full scale batches, thus saving time and expense.
While controlling the process and optimizing the formulation during scale up, Oakwood provides all necessary CMC documentation for regulatory filings. Due to our extensive quality system, we ensure that the pharmaceutical scale-up process has successfully passed CMC reviews by regulatory authorities.
Ensure Quality with Our Scale-Up Process
With our in-house quality systems, we offer quality assurance and validations through pharmaceutical scale-up. At Oakwood Labs, we have an extensive process for our systems to go through in order to be verified through our quality control department. We have illustrated our commitment to quality with our proven history of compliance.
Contact Us Regarding Our Scale-Up Process
Oakwood Labs has been a premier resource for all phases of development for long-acting injectables since 1997. We are an industry leader in sustained-release injectable pharmaceutical development and manufacturing. We are committed to products that provide enhanced therapeutic benefits to patients.
Contact us to learn more about our scale-up process or our other manufacturing capabilities.

