Partnering Opportunities
Partnering Opportunities
In addition to working with external partners who are seeking a long acting injectable (LAI) product being developed for them, Oakwood also embarks on internal programs using our in-house microparticle technology. Oakwood’s business development team performs extensive market research along side key opinion leaders (KOLs) to determine unmet needs within the market that would be solved with the use of Chroniject™. These opportunities often arise from the ability to convert a product to a LAI formulation – or to take a current program and tailor the target product profile to provide a longer therapeutic duration of release that what exists currently. Both of these factors provide with significant advantages in terms of achieving both patient compliance and convenience. An LAI product would command a premium price even after the expiration of its patent due to the difficulty in developing generics and can be used to extend the exclusivity of oral products nearing the end of their patent lives.
For these internal programs, Oakwood performs all feasibility development activities in-house to confirm proof of concept. Often initial formulations are confirmed in vitro as well as in vivo with the use of a rat or dog study. After confirming feasibility, the next step is to perform optimization of the formulation and scale up to the batch size required for a Phase I clinical trial. Scaling-up the batch size is a critical development activity, which requires a large amount of experience and know-how. Oakwood’s technology specializes in solving this problem and provides a competitive advantage to other drug delivery platforms.
At this point in the development process Oakwood actively seeks a external partner for co-development of the program. The external partner would be responsible for funding the on-going development activities, Phase 1-3 clinical trials, and registration/commercialization efforts. During the due diligence process of partnering the program, Oakwood will supply the partner with detailed information related to market opportunity, rationale for the program, development work to date, and estimated financial projections.
Therapeutic Areas of Interest
Oakwood’s technology allows for the development of products in any therapeutic area in which there is a need, and currently supports its programs in the following markets:
- Central Nervous System
- Oncology
- Endocrinology
- Opioid and Substance Abuse
- Joint Pain
- Cardiology
- Post-Operative Pain
Central Nervous System
Oakwood has successfully manufactured and scaled up formulations with a wide array of APIs commonly used in the CNS space. The first-line treatment in this space is commonly oral medication. Due to adherence and compliance issues observed in many patients, an LAI product would provide a major upgrade to current treatment options. The patient would be given the product at a clinic by a healthcare professional. Oakwood is currently developing products focused on treating schizophrenia, bipolar disorder, and depression with release durations of one to two months
Oncology
Oakwood has used its technology to develop formulations focused on treating mantle cell lymphoma, small lymphocytic lymphoma, chronic lymphocytic leukemia, and multiple myeloma with release durations ranging from two to four weeks. The use of an LAI product would allow for a reduction in the total amount of drug administered to the patient, as well as less frequent doses. Due to these reductions, there would be less adverse reactions and side effects, greatly benefiting patients.
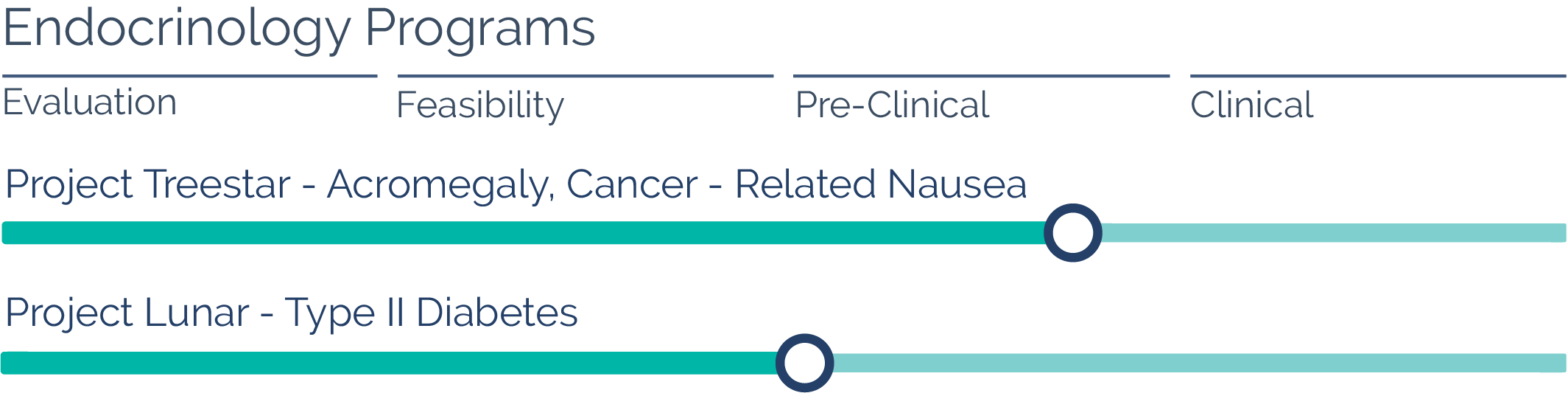
Endocrinology
Oakwood has successfully manufactured and scaled up formulations focused on treating acromegaly and carcinoid tumors. The daily injections initially used for these conditions have been largely replaced by Sandostatin LAR, a one-month LAI product currently available in this space. Oakwood’s two-month formulations would further reduce the number of injections and office visits by 50%, which could enhance patient compliance.
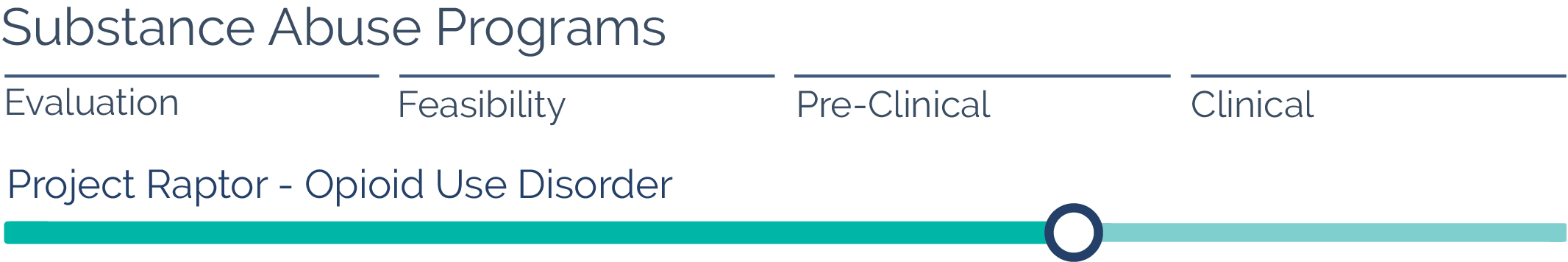
Substance Abuse
Oakwood has successfully manufactured and scaled up formulations focused on treating opioid use disorder. The first-line treatment in this space involves the use of opioid replacement therapies. Although these have been primarily oral medications in the past, there is now a commercially available one-month LAI product. Oakwood has developed formulations that have significant advantages over the commercially available one-month LAI product. Oakwood’s two-to-four-month formulations would reduce the number of injections and office visits, which could enhance patient compliance.
Joint Pain
Oakwood has used its technology to develop formulations focused on treating osteoarthritis and rheumatoid arthritis with a release duration of three months. An LAI product would be extremely useful in this space because it can be used to target a specific anatomical area. Due to the ability to maintain higher drug concentrations in a targeted area, systemic exposure would be reduced. A product with higher localized drug levels at the targeted site and reduced side effects would be advantageous to other treatment options and may be disease modifying.
Cardiology
Oakwood has used its technology to develop formulations focused on treating pulmonary arterial hypertension with a release duration of one month. Current treatments, which are mostly oral medications, work in different ways but ultimately attempt to decrease symptom severity and increase exercise capacity. The use of an LAI product would offer benefits similar to other therapeutic areas including reduction in the total amount of drug administered to the patient, as well as less frequent doses.
Post-Operative Pain
Oakwood has successfully manufactured formulations that can be used for post-operative pain. The levels of pain caused by surgeries can vary greatly depending on the type of surgery and how individual patients tolerate and respond to pain. Medication is given as needed, with amounts and strengths being carefully determined. An LAI approach would involve patients receiving a single injection following an operation, which can release in a matter of days or weeks. This approach would prevent any potential for abuse.
Contact Our Business Development Team For More Information
If you have a interest in any of the long acting injectable programs mentioned above please reach out to our business development team today by emailing bd@oakwoodlabs.com or filling out a contact form on our website. We look forward to providing you more detailed information and furthering the partnering conversation.